#NephMadness 2021: Animal House Region
Submit your picks! | NephMadness 2021 | #NephMadness | #AnimalHouse
Selection Committee Member: Kelly Hyndman @DrKeeksPhD
Kelly Hyndman is an Assistant Professor of Medicine in the Section of Cardio-Renal Physiology and Medicine, Division of Nephrology, at the University of Alabama at Birmingham. She has trained both in comparative physiology and kidney physiology labs and is currently a principal investigator of a basic science lab with research interests in novel mechanisms of fluid-electrolyte balance.
Writer: Tiffany Truong @CRRTiff
Tiffany Truong is a 2nd year nephrology fellow at the University of Southern California. Her academic interests include transplant nephrology, electrolyte disorders, medical education, and finding creative ways to better advocate for her patients.
Competitors for the Animal House Region
Hopping Mouse vs Marine Iguana
Seahorse vs Hagfish

Copyright: Maridav / Shutterstock
Recognizing that we have the kind of internal environment we have because we have the kind of kidneys we have, we must acknowledge that our kidneys constitute the major foundation of our physiological freedom. Only because they work the way they do has it become possible for us to have bones, muscles, glands and brains. Superficially, it might be said that the function of the kidneys is to make urine; but in a more considered view one can say that the kidneys make the stuff of philosophy itself.
Homer Smith, From Fish to Philosopher
If one needs proof of the power of comparative nephrology, look no further than the origins of kidney physiology. It is the stuff of legend, and an origin story for nephrologists everywhere. Dim the lights and cue the music as we begin our story along the coast of Maine, in a lab fantastically named Mount Desert Island (MDI) Biological Laboratory. It is here that E.K. Marshall and Homer Smith used their experiments with fish to elucidate basic principles in tubular transport, glomerular filtration, and renal blood flow. As stated in Krogh’s principle, “For many problems there is an animal on which it can be most conveniently studied.” Indeed, the study of animals and their evolutionary adaptations has allowed us to understand how more complex systems came to be, to model physiology and pathophysiology, and to develop our scientific methods and tools. To this day, humble nephrology trainees migrate to MDI annually to learn the “Origins of Renal Physiology” through experiments with classic physiology model systems including the toad’s bladder, the shark’s rectal gland, and the zebrafish. As we trace the elaborate and still mystifying ways that living beings regulate their internal environments, we embark on a journey to celebrate the physiologic diversity of living organisms that have adapted to survive in unique and often desolate landscapes.

The evolution of vertebrates in saltwater (dark blue) freshwater (light blue), and land (brown). Note the aglomerular fish that have migrated back to saltwater in the bottom right. Adapted by Matthew Sparks and Corina Teodosiu from Hickman CP Jr, Trump BF. The Kidney. In: Hoar WS, Randall DJ, eds. Excretion, Ionic Regulation, and Metabolism. Academic Press, Inc; 1969:91-239. Fish Physiology; vol 1.

The Kidney Shed at MDI as depicted in this original Renal Fellow Network post
Our first matchup is a battle between two animals who have learned to survive, despite free water deprivation: the spinifex hopping mouse (Notomys alexis) of the Australian desert, and the Galápagos marine iguana (Amblyrhynchus cristatus) who thrives by the sea. How have these animals adapted to landscapes devoid of free water?
Hopping Mouse vs Marine Iguana
Hopping Mouse
Muad’Dib is wise in the ways of the desert. Muad’Dib creates his own water. Muad’Dib hides from the sun and travels in the cool night. Muad’Dib is fruitful and multiplies over the land.
Frank Herbert, Dune

Picture (L) courtesy of Pippa Kern / Bush Heritage Australia. Picture (R) courtesy of Annette Ruzicka / Bush Heritage Australia. Used with permission.
The hopping mouse has adapted to live in an environment without much access to water, with the kidney playing an essential role in that adaptation. The kidney of a hopping mouse can concentrate its urine more than 20-fold, making it the most concentrated urine among mammals at a whopping 9,000 mOsm/kg. The Hopping mouse can achieve this by its power packed loops of Henle, arranged to allow for a massive interstitial gradient from cortex to papilla. Thus, the hopping mouse is in constant search for water. With an adequate supply of water, nobody is likely to beat Team Hopping Mouse.
It may come as no surprise that our first spectacular animal is one of many bizarre and unique inhabitants of the wild arid Australian terrain. How does a humble mouse make this list? The spinifex hopping mouse holds the distinction of producing the most highly concentrated urine ever recorded from a mammal, topping out at a whopping 9,370 mOsm/kg (compared to human urine ranging 50-1200 mOsm/kg)! Some have described this mighty mouse’s urine as so concentrated that it is practically solid. Its urine can reach levels of concentration more than 20 times greater than that of its own plasma, making it a fascinating model for the study of mechanisms of urinary concentration and the creation of very high osmotic gradients in the kidney.
Mechanisms to conserve water expand beyond the kidney. The spinifex hopping mouse also reduces fecal water content, and avoids water loss to heat by being nocturnal and inhabiting burrows. It maximizes the generation of “metabolic water”, which is water created through the metabolism of its food. When deprived of water, the spinifex hopping mouse has increased appetite to provide a substrate for metabolic water production and will also store consumed carbohydrates as glycogen rather than fat, which generates less metabolic water. The spinifex hopping mouse may be one of the animals most successfully adapted to the arid environment of the desert.
The kidney of the spinifex hopping mouse can be appreciated for the ways it has structurally and physiologically adapted to its arid environment. Desert rodents have longer water-permeable descending thin limb segments, and produce higher levels of vasopressin than typical lab rats during times of water deprivation. The adaptations to conserve water don’t stop there. Studies in spinifex hopping mice have also demonstrated differential control of atrial natriuretic peptide (ANP) expression. ANP synthesis in the kidney is responsible for regulating fluid and electrolyte balance in response to water deprivation, salt loading or diabetes. Finally, it has been proposed that water deprivation in the spinifex hopping mouse decreases hyaluronan synthesis. This decreases the viscosity of the interstitial matrix and its ability to bind water, allowing greater water efflux from the descending loops of Henle and the collecting ducts.
The marked ability of the spinifex hopping mouse to conserve water allows it to serve as a model to untangle mechanisms of urinary concentration which might be generalizable to other organisms. What is known and what remains unknown about mechanisms of urinary concentration? When we think about concentrating urine, we picture the hormone arginine vasopressin (affectionately called “antidiuretic hormone”) promoting the insertion of water channels called “aquaporins” into principal cells of the collecting duct, to ultimately drive water reabsorption from the tubular filtrate. To achieve this, an interstitial osmotic gradient allowing water reclamation must exist. Recall that in the outer medulla, the descending thin limb of the loop of Henle is highly permeable to water and urea, initially concentrating the ultrafiltrate as it transits the tubules. Then, the thick ascending limb of the loop of Henle actively reabsorbs sodium chloride (removing it from the ultrafiltrate) while blocking water efflux, thereby diluting the ultrafiltrate and concentrating the interstitium. Due to the direction of flow, countercurrent multiplication occurs, forming the interstitial osmotic gradient needed to drive water reabsorption in the collecting duct. This process is driven by the small osmolality difference between the ascending and descending limbs created by the active transport of sodium chloride in the ascending limb, which has been termed the “single effect”. It is multiplication of the “single effect” by countercurrent flow in the branches of the loop of Henle that gives rise to the larger interstitial gradient from the cortex to the papilla that is needed to concentrate urine.

Visual abstract by Corina Teodosiu on Pannabecker et al.
In the inner medulla the osmolality gradient continues, but the source (or “single effect”) remains controversial as perfused tubule experiments demonstrated no significant active NaCl transport in thin ascending limbs. Study of the spinifex hopping mouse (and of other desert rodents) that are highly adapted to concentrating urine may give us insight into these mechanisms. One hypothesis first proposed in 1972 is the “passive mechanism”, whereby urea concentrated in the interstitium via collecting duct urea transporters contributes to the osmolality gradient of the medullary interstitium. We now know that urea makes up approximately 50% of the solutes in the interstitium that maintain the osmolality gradient. Moreover, this process decreases the NaCl concentration in the interstitium relative to that in the thin ascending limb after osmotic equilibration, creating a gradient for NaCl to diffuse into the interstitium, and potentially creating the single effect in the inner medulla. Though the passive mechanism, as it was first proposed, provided a plausible conceptual model, there were some discrepancies with mathematical modeling. Since the 1990s, multiple alternatives to the passive mechanism have arisen including: 1) enhanced detail of medullary anatomy and transepithelial transport to comprehensively explain an effective passive mechanism, 2) different single effects generated in collecting ducts or thin descending limbs, and 3) pelvic peristaltic contractions which mechanically impact water flux in the medullary papilla.
Another point to note about urinary concentration is the ability of rodents, such as the spinifex hopping mouse, to concentrate their urine more effectively than large animals. It is thought that animals with greater medulla-to-cortex area and long thin loops of Henle are able to concentrate their urine better. Compared to spinifex, most mammals have a diminished medullary area. This has also been observed in mice with deletion of genes in the renin angiotensin system, where removal leads to a diminished urinary concentrating ability and smaller medullary structure of the kidney.

Medullary size is correlated with urinary concentration. Made by Matthew Sparks. Adapted from Withers PC. Comparative Animal Physiology. Saunders College Publishing; 1992. Created with Biorender.com.
Osmotic gradients aside, no discussion of urinary concentration in the face of severe water deprivation would be complete without discussing the role of aquaporins themselves. In the desert rodent, we see aquaporins across all the organ systems. In the descending thin limb of the loop of Henle, water is chiefly reabsorbed through aquaporin 1 (AQP1), which is notably abundant in the initial segment of the descending thin limb and nearly absent in the more distally deep inner medulla. In another desert rodent, the kangaroo rat, this segment is a much longer proportion of the inner medulla than in the typical lab rat, possibly allowing for greater water reabsorption in this animal. The AQP1-negative segment, on the other hand, is permeable to small solutes such as sodium and urea. Thus, different segments of the thin descending limbs in the medulla contribute differently in producing the corticomedullary osmotic gradient. In the inner medullary collecting duct, water flux through aquaporins play an even more central role via aquaporin 2 (AQP2) on the apical side of principal collecting duct cells, and aquaporin 3 (AQP3) and aquaporin 4 (AQP4) on the basolateral side. During water deprivation, vasopressin increases AQP2 trafficking to the apical membrane. Studies in rats have shown that most increases in urine osmolality occur in the last 25% of the rat medullary collecting duct, and this was due entirely to water reabsorption. In the spinifex hopping mouse and other rodent models, we have seen that AQP2 expression is also regulated by hypertonicity of the interstitium via the transcription factor tonicity-responsive enhancer binding protein (TonEBP), apparently independently of vasopressin, and also in response to water deprivation.

Relative differences in length of loop of Henle from A. Nephron structure from a typical mammal. B. Nephron structure with longer length of loop of Henle in a Hopping Mouse. Made by Matthew Sparks. Created with Biorender.com.
Marine Iguana
Water, water, every where,
Nor any drop to drink.Samuel Taylor Coleridge, The Rime of the Ancient Mariner

Copyright: Kim de Buiteleir / Shutterstock
The marine iguana has a conundrum: It is surrounded by seawater and not much fresh water. Sadly, the marine iguana did not develop its loops of Henle and thus needs an alternative way to get rid of the excess salt in order to maintain their plasma osmolality. To do this, reptiles (and marine birds) have adapted to their environment by using salt glands to excrete hypertonic salt rich fluid. The marine iguana’s salt gland is located just above the orbits and is connected to the nasal passages, allowing it to “sneeze” salt.
Although it may appear that marine reptiles such as the Galápagos marine iguana are surrounded by water, the environmental challenges they face are similar to those of the spinifex hopping mouse in that there is limited free water and an excess of salt which must be excreted. Reptiles do not use their kidneys to handle this salt excess, as they do not have a loop of Henle (bummer) and so cannot produce urine that is hyperosmolar to plasma like mammals. Because of this limitation, many reptiles have an extrarenal salt-secreting organ. In the Galápagos marine iguana, this salt gland and excretor is located above the orbit and is connected to the nasal passages so that their salty secretions are forcefully expelled through the nostrils. Sounds a lot like sneezing salt, right? See it for yourself here.

Differences in sodium content in the salt glands of various reptiles and birds as compared to seawater. Made by Matthew Sparks and Corina Teodosiu.
Among reptiles, the Galápagos marine iguana distinguishes itself as the only lizard that feeds in the sea, diving to obtain its diet of mostly algae, which contain high concentrations of electrolytes including sodium and potassium. Since what goes in must come out, the degree of electrolyte excretion by the Galápagos marine iguana is, as expected, very high to match, with the salt gland being the major route of excretion of sodium, potassium, and chloride. In fact, the salt gland of the Galápagos marine iguana has the highest known secretory rate of sodium and potassium of any reptile. The iguana secretes so much salt that it is frequently seen wearing a white crown of crystallized salt encrusting its head and snout. This salt gland also has the lowest known Na/K ratio of any marine reptile, secreting more potassium than other reptiles. Other marine animals do possess extrarenal salt glands, including sea snakes (in the tongue), marine birds (at the tear duct), sharks (in the rectum), and sea turtles (by the eye). The salt glands of these marine animals are useful models of active electrolyte transport.

Visual abstract by Sophia Ambruso on Schmidt-Nielsen.
Although the exact details of the marine iguana’s salt gland are still a mystery, we can hypothesize that it may be similar to that of the marine bird. In gulls, the salt gland is about 0.1% of the body mass. By comparison, the mammalian kidney is about 1%. However, the gland of the gull can secrete 0.5 mL/min per gram of glandular tissue, compared to the glomerular filtration rate (GFR) of a human, which is 0.2 mL/min per gram kidney. Pretty impressive that a salt gland can secrete at a rate higher than our GFR!
You might be wondering at this point whether reptilian salt glands are also responsible for the legendary “crocodile tears” produced as crocodiles eat their prey (the term is used to connote false displays of grief). Alas, studies have shown that crocodile tears are not the byproduct of salt glands; their salt glands, located in 1981, were found by the tongue. Nonetheless, the syndrome of gustatory lacrimation in humans recovering from facial nerve palsy has been given the moniker “Crocodile tears syndrome”. In this spirit, it is a small wonder that exuberant sneezing in humans has yet to be similarly termed “iguana sneezes”.
Seahorse vs Hagfish
When is a kidney not a kidney? In this next matchup, we explore wild forms of what we define as a kidney with the aglomerular seahorse and the primitive osmoconformer, the hagfish.
Seahorse
What engineer, wishing to regulate the composition of the internal environment of the body…, would devise a scheme that operated by throwing the whole thing out sixteen times a day – and rely on grabbing from it, as it fell to the earth, only those precious elements which he wanted to keep?
Homer Smith, From Fish to Philosopher

Copyright: GOLFX / Shutterstock
The seahorse has no glomerulus. That’s right all of you glomerular aficionados, nothing to see here! This is a tubule only zone—no gloms allowed. How in the world can a seahorse live without precious glomeruli? Well, it turns out, tubules are more powerful than you think. Although the seahorse lacks gloms, they are able to produce urine with a similar composition to fish with glomeruli. This is achieved by an impressive array of tubules that run counter current to a renal portal system allowing for the creation of an ultrafiltrate. Thus, the tubules of the seahorse have impressive capabilities. Who needs glomeruli anyway?!
Seahorses are any of the 46 species of marine fish within the genus Hippocampus, derived from the Greek words hippos meaning “horse” and kampos meaning “sea monster.” In ancient times, they were thought to be related to the mythical horses of the sea which drew Poseidon’s chariot. Legends aside, the seahorse is full of surprises. From having eyes that can look both forward and backward at the same time to snouts that suck up food like a vacuum cleaner, there appears to be no end to the spectacular qualities of this colorful animal. But in the context of NephMadness, perhaps no feature is more captivating than the seahorse’s lack of glomeruli.

The aglomerular kidney of the seahorse. A. Black line indicating the cross section that is depicting kidney structures in panel B. B. Blood flows from the caudal vein to the large intrarenal vein bathing each of the tubular segments by the renal portal system. C. Urine is formed through secretion of solutes and ions from the portal system to the tubular fluid which is emptied into the mesonephric duct to the ureter. Made by Matthew Sparks and Corina Teodosiu. Created with biorender.com
Why do glomeruli exist? This was the nephro-existential question that confronted scientists who discovered the completely aglomerular kidney of marine fish such as the goosefish, the toadfish, and the seahorse. To answer this question, we first need to understand what tubules are capable of on their own. At the time of the discovery of the aglomerular kidney, scientists were caught in what has been called an “acerbic controversy” about whether tubules could secrete waste products on their own or if they were limited to simply reabsorbing elements that had already been filtered by the glomerulus. Aglomerular kidneys were the model which demonstrated that tubular secretion is not only a reality, but that the tubules are fully capable of secreting most constituents of urine. In one of the earliest studies comparing aglomerular and glomerular kidneys, E.K. Marshall reported that aglomerular fish had urine flow comparable to that of glomerular fish. Surprisingly, the composition of urine in terms of electrolytes and nitrogenous waste products was also similar among all of the different fish species, glomerular or not. When it comes to the excretion of foreign substances (not produced by the organism’s body), however, glomerular kidneys can excrete nearly all water-soluble substances, while aglomerular kidneys seem to exhibit selectivity, more similar to a secretory gland that one might find in the digestive system.
So do we need glomeruli at all if tubules alone are able to excrete urine of similar composition and with similar flow rates? Homer Smith postulated that during vertebrate evolution in freshwater, glomeruli evolved because filtration helped to excrete the large volume of water absorbed from the freshwater environment. When the early fishes were pushed out of freshwater and back into the marine, salty environment, excess water was no longer an issue and the major advantage of a filtration system was lost. Thus, in the ancestors of the seahorse, glomeruli began to shrink, and in certain species disappeared entirely. Birds and mammals which could adapt to arid environments in land and air, on the other hand, kept their glomeruli and developed a new structure to finally be able to secrete a hypertonic urine—the loop of Henle.
Just as important as what tubules can secrete are what they have been shown not to excrete—chiefly, carbohydrates and protein, the valuable energy sources and building blocks of living organisms.
Glucosuria could be induced in glomerular fish by producing hyperglycemia and administering phlorizin, a competitive inhibitor of sodium/glucose cotransporters 1 and 2 (SGLT1 and SGLT2), but this could not be reproduced in aglomerular fish. Understanding that tubules would not actively secrete carbohydrates contributed to the historic development of the use of the carbohydrate inulin to measure GFR. The aglomerular kidney thus functions as a natural knockout model. Without the aglomerular kidneys of seahorses and other marine creatures, we would not be where we are today in our understanding of both tubular secretion and glomerular filtration.
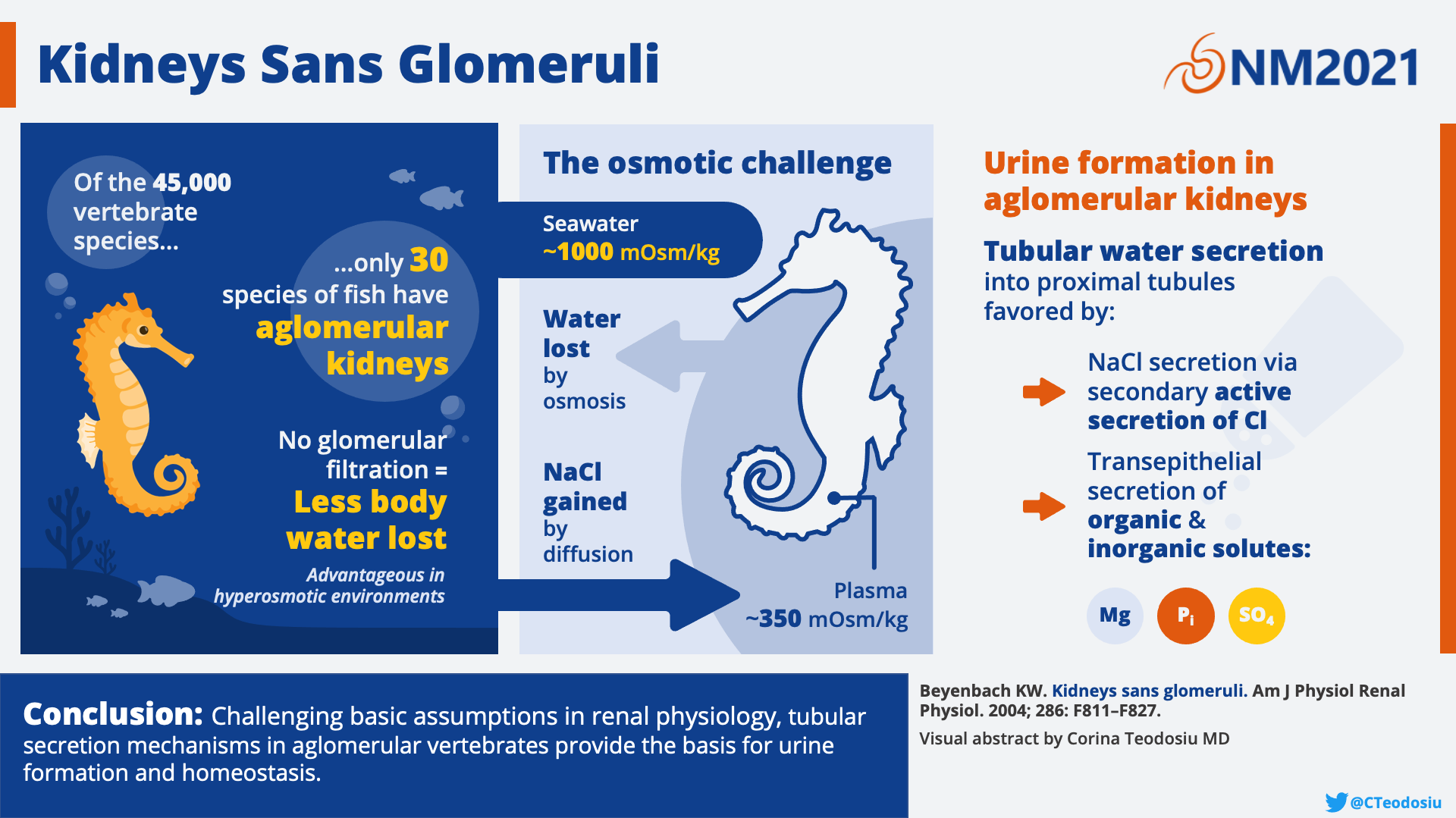
Visual abstract by Corina Teodosiu on Beyenbach et al.
Finally, another development that evolved through the study of aglomerular fish was the use of para-aminohippuric acid (PAH). To measure renal blood flow, a compound is needed which is completely excreted as it passes through the kidneys to reflect the blood flow. This requires that it is not only filtered by the glomerulus, but also secreted by the tubules. After observing the avid excretion of organic acids in aglomerular fish, PAH was found to be a suitable marker.
The achievements of our predecessors should not be the end of what we can learn from aglomerular fish such as the seahorse. Today, we consider the loss of GFR to be synonymous with kidney failure, but aglomerular fish have no glomerulus (and consequently, no GFR) and are not in kidney failure. It has been proposed that the kidney tubules of aglomerular and glomerular kidneys are quite alike, and that the difference between glomerular and aglomerular urine formation is “one of degree rather than of kind”. Even in fish that have glomeruli, glomerular filtration may at times completely cease in some nephrons, a phenomenon called glomerular intermittency, and this interruption does not equate to kidney failure. Have we underestimated the role that tubules play in maintaining our internal environments? Can we use these animals to discover new strategies to treat or identify kidney disease? The seahorse is a fierce contender in NephMadness, given its historical significance and the hope that aglomerular kidneys represent in our understanding of kidney physiology.
Hagfish
Hagfish are widely considered the most disgusting animals in the ocean, if not on earth.
Hannah Waters, SmithsonianMag.com

Picture (L) courtesy of Dr. Alyssa Weinrauch. Picture (R) courtesy of Dr. Dean Grubbs / Florida State University, common species found in the Gulf of Mexico. Myxine mcmillanae (top), Eptatretus minor (middle), Epatretus springeri (bottom). Used with permission.
Please don’t Google the Hagfish. You need to be prepared for what you will see! The hagfish is considered the most primitive living vertebrate. They scavenge the sea, feeding on the carcasses of larger animals. The hagfish has adapted by becoming one with its salty environment. No salt gland? No problem! With a serum osmolarity of 1000 mOsm/L, it is similar to seawater. Because it does not need to even mess with the energy rich task of osmoregulation, the hagfish requires less energy, another peculiarity of the hagfish is its enormous glomeruli (about 10x larger than mammals). Take that, seahorse!
Finally, we turn our attention to the hagfish, a scavenger of the sea who burrows into the carcasses of larger animals to feed and produces copious amounts of a unique, sticky slime to defend itself.
Hagfish are considered the most primitive living vertebrates, “the ‘cradle’ of our vertebrate origins”. Their development is so obscure that whether or not they should even be considered vertebrates (as they don’t have vertebrae as adults) is controversial. As an evolutionary bridge between vertebrates and invertebrates, hagfish resemble invertebrates in many ways. Unlike many other marine vertebrates, hagfish are osmoconformers, with a serum osmolarity that is similar to the surrounding seawater (>1,000 mOsm/L). Even more distinguished, they achieve this high osmolarity by having a nearly identical ionic composition to the sea. In other words,from an electrolyte point of view the blood plasma of a hagfish is essentially seawater! This contrasts with sharks and other cartilaginous fish, which achieve a seawater-like plasma osmolarity using high levels of urea (recall from NephMadness 2018 that the urea concentration of a shark’s plasma is 1,300 mg/dL) and other organic osmolytes. Hagfish are the only vertebrates that do not regulate the exchange of monovalent ions and water with their environment. The ions that are regulated (through unknown mechanism) are the divalent ions (Mg2+, Ca2+, SO42-). That this peculiar internal environment can nonetheless support the cellular functions of life in the hagfish is certainly a marvel, and one that bestows upon them a particular advantage. Requiring little energy for osmoregulation, hagfish have among the lowest metabolic requirements of any vertebrate, helping them to survive severely hypoxic, or even anoxic, environments.
Why might it be important for the hagfish to tolerate hypoxia? Charmingly, the hagfish is a bottom-dwelling opportunistic feeder which needs to burrow into the harsh depths of decaying marine animal carcasses for its meals. Not only does the hagfish encounter low oxygen levels amidst its feasting, it also must deal with all of the ammonia and urea being released from its dinner. Which brings us to our next point; the hagfish is exposed to very high amounts of nitrogenous waste from the water surrounding it while it is eating, as well as from its own metabolising of its high protein diet. Most fish excrete their nitrogenous waste primarily through their gills rather than through their kidneys, and typically this nitrogenous waste is excreted in the form of ammonia (rather than urea, as in mammals). The hagfish is no exception here. Why do animals excrete nitrogen in different forms? Ammonia is very energetically cheap to make (costs very little ATP), but is highly toxic. Urea, on the other hand, is metabolically expensive, but is less toxic. Like other fish, the hagfish lives in an environment where plenty of water is present to wash away the toxic ammonia, allowing them to conserve ATP. We land-dwellers don’t have enough water to prevent ammonia toxicity, so using ATP to make urea is a better deal for us. The hagfish does have one particular quality that distinguishes it from other fish in terms of nitrogenous waste handling; the hagfish is able to actively excrete ammonia across its skin. This is not simple diffusion. As the hagfish is often faced with a large amount of ammonia in its environment, it must excrete against a concentration gradient. This additional mechanism of nitrogenous waste excretion may reflect how uniquely the hagfish has adapted to environments with high ammonia and urea. Another surprising quality of hagfish skin is its ability to absorb amino acids, doubling its duty as not only an excretory organ, but also as an organ for nutrient acquisition. The curious properties of the hagfish skin, as well as its tolerance of unusually high levels of plasma ammonia and urea, make the hagfish an interesting prototype of nitrogenous waste handling.
While the cells of the hagfish must adapt to a pretty wild internal environment (high NaCl, high ammonia), maintaining this internal environment seems relatively straightforward, as it mimics the external environment. With the gills and skin in play as well, what exactly does a hagfish kidney even need to do?

The developing nephron. Figure 1 from Romagnani et al. Used with permission.
As you might expect, a hagfish kidney is quite primitive. Recall from embryology that humans develop three different “kidneys” or excretory organs throughout life, each more complex than the one it replaces, and each representing different stages in evolution. Humans start with the pronephros, then move onto the mesonephros, and finally the metanephros, which becomes the adult kidney in humans. Aquatic vertebrates typically do not ever develop a metanephros, and their kidneys are a functional mesonephros (also called an “opisthonephros” when this is the final evolutionary stage). The hagfish keeps its pronephros, sometimes called its “head kidney”, but functionally uses the mesonephros as its kidneys. The hagfish kidneys are paired structures, each composed of ~35 very large, segmentally arranged glomeruli with Bowman’s capsules connected by short necks directly into the archinephric duct, which then drains into the cloaca. The archinephric duct is lined with epithelial cells and has a brush border which resembles the proximal tubule.

Hagfish kidney. Left, macrostructure of the hagfish kidney, Right, nephron showing glomerulus where plasma ultrafiltrate is made and archinephric duct (or tubules) where secretion and reabsorption occurs. Adapted by Matthew Sparks and Corina Teodosiu from Hickman CP Jr, Trump BF. The Kidney. In: Hoar WS, Randall DJ, eds. Excretion, Ionic Regulation, and Metabolism. Academic Press, Inc; 1969:91-239. Fish Physiology; vol 1.
Despite its simplicity, the hagfish kidney does share some similarities with that of mammals. Glomerular filtration barrier characteristics are similar, the archinephric ducts have sodium cotransport systems and can reabsorb glucose like the proximal tubule, and it can concentrate magnesium, phosphate, sulfate, and likely hydrogen ions. All of these traits allow the hagfish kidney to serve as a useful model of kidney physiology and pathophysiology. Not to mention, the hagfish glomeruli are enormous (500-1500um, about ten times larger than most mammals), making them easy to dissect and use in isolated glomerular studies. Isolated hagfish glomeruli have been used to study the effect of hormones or hemodynamic changes on glomerular filtration, and to demonstrate mechanisms of toxicity of heavy metals such as cadmium, or medications such as adriamycin.

Visual abstract by Sophia Ambruso on Currie et al.
The hagfish may not be a looker or a charmer, but it certainly plays an important role as perhaps the most primitive living vertebrate with primordial “kidneys”, which serve as a useful model as well as evolutionary memento. With a unique osmoconforming internal environment that resembles the surrounding sea, the hagfish also gives new meaning to the phrase “going with the flow.”
COMMENTARY BY KELLY HYNDMAN:
The evolution of the nephron – lessons from the wild
– Executive Team Member for this region: Matthew Sparks, AJKD Social Media Advisory Board member. Follow him @Nephro_Sparks.
How to Claim CME and MOC
US-based physicians can earn 1.0 CME credit and 1.0 MOC point for reading this region.
- Register/log in to the NKF’s Professional Education Resource Center (PERC). If you select “Physician” in the drop-down menu during registration, the ABIM ID will pop up – make sure to complete this during registration to receive MOC points after course completion.
- Review the activity, disclosure, and accreditation information.
- Click “Continue” and review Course Instructions.
- Complete Post-Test. Please note: By selecting “Yes” to the participation questions for each region, the corresponding Post-Test questions will appear. Click “Save Draft” to save your responses and finish later. When you are ready to submit your answers, click “Preview” to review all responses, then click “Submit.”
- Click “Next” to complete the Evaluation form, then click“Submit.”
- Claim 1.0 CME credit and 1.0 MOC point per region (up to 8.0 total for 8 regions of NephMadness).
- Save/print your certificate.
The CME and MOC activity will expire on June 14th, 2021.



Leave a Reply