NephMadness 2016: Missteps in Nephrology Region

Submit your picks! | For more on NephMadness 2016 | #NephMadness or #MisstepsRegion on Twitter
We like to think that medical progress flows in one direction, like a river. Over time we learn and provide more evidence-based therapies so that our patients continue to get better. When we pull back to a high-enough altitude, that is exactly what we see, the invention of IV fluids then antibiotics, and vaccines, then primitive cancer therapies followed by more advanced ones. But when you start to really focus in, the details look more chaotic. Misinterpretations of the data can send a specialty hurtling down a dead end and it can take decades for the standard of care to recover. Some of these missteps are an inevitable byproduct of trying to stay on the cutting edge, but when they occur we should take a time to understand how we went wrong so as not to make the same mistake the next time; we can learn as much from our failures as we do from our successes.
Selection Committee member for the Missteps in Nephrology Region:
Mark Rosenberg, MD
Mark Rosenberg is Professor of Medicine, and Vice Dean for education at the University of Minnesota Medical School. In this role he oversees the continuum of medical education. His leadership promotes innovation of teaching methods and continuity of curriculum from pre-med and undergraduate through graduate and continuing medical education. He serves as an ASN Councillor and Chair of the ASN Education Committee and will be ASN President in 2019. He is the recipient of the 2013 Eric G. Neilson, M.D., Distinguished Professor Award from the Association of Specialty Professors for his outstanding leadership in the area of specialty medicine and the 2013 Robert G. Narins Award from the American Society of Nephrology for his contributions to nephrology, education, and teaching. His research interests focus on the pathophysiology of both acute and chronic kidney disease and more recently on telehealth as a way of managing patients with chronic diseases.
Meet the Competitors for the Missteps in Nephrology Region
Normalization Hemoglobin
ACEi ARB combo
Aluminum Binders for Hyperphosphatemia
Steroids for Idiopathic Membranous
Normalization Hemoglobin vs ACEi ARB combo
Normalization of Hemoglobin
 One of my favorite questions to ask notable nephrologists is, “looking back over your career, what has been the most revolutionary change in nephrology?” The answer repeatedly, has been the development and wide availability of epoetin alfa. Epoetin came to market in 1989 and within a year revolutionized the experience of dialysis. Transfusions among dialysis patients were so common that USRDS measured transfusions per three months rather than transfusions per year. Epoetin completely transformed this experience. In the space of one year EPO knocked the transfusion rate down by more than half and the transfusion rate continued to fall. Towards the end of 2000 it fell below 1% per three months.
One of my favorite questions to ask notable nephrologists is, “looking back over your career, what has been the most revolutionary change in nephrology?” The answer repeatedly, has been the development and wide availability of epoetin alfa. Epoetin came to market in 1989 and within a year revolutionized the experience of dialysis. Transfusions among dialysis patients were so common that USRDS measured transfusions per three months rather than transfusions per year. Epoetin completely transformed this experience. In the space of one year EPO knocked the transfusion rate down by more than half and the transfusion rate continued to fall. Towards the end of 2000 it fell below 1% per three months.

Reproduced from the 2008 USRDS ADR.
But by this time physicians were targeting a different end-point. In the introduction to Besarab et al’s landmark study of normalization of hematocrit published in 1998, there is no mention of transfusions in introduction or rationale for the study. The goal of increasing hemoglobin was to reduce mortality and heart attacks. The trial failed to reduce mortality or heart attacks and the untrained eye may be concerned by what appeared to be an increased rate of the primary end-point in the normal hematocrit group:
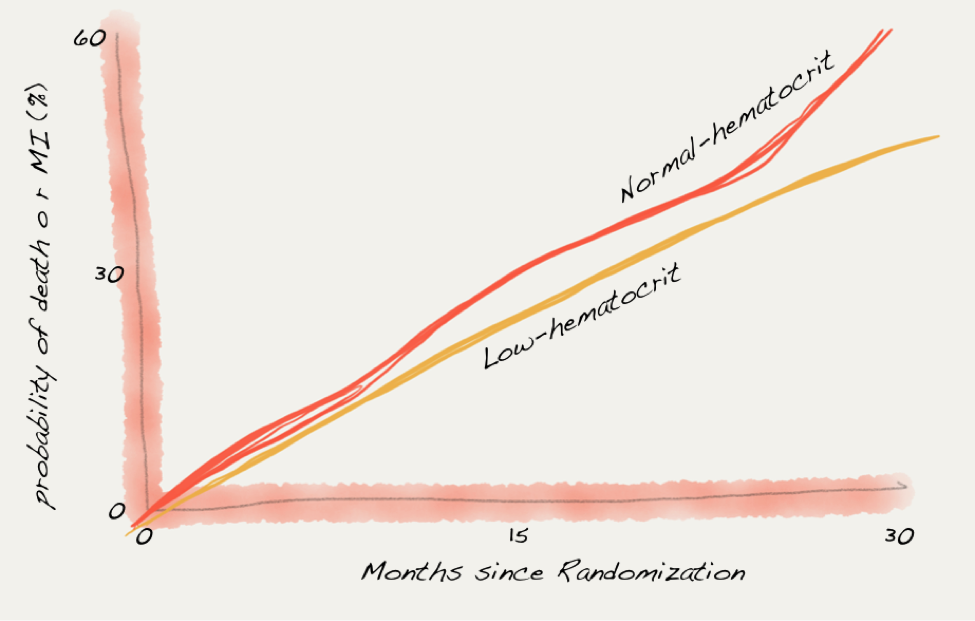
Data from Besarab et al.
But experts will be reassured by the 95% confidence interval crossing the line of identity: “risk ratio for the normal-hematocrit group as compared with the low-hematocrit group, 1.3; 95 percent confidence interval, 0.9 to 1.90.”
The study was stopped early by the data safety monitoring board despite not reaching the prespecified 5% level of significance. I wonder if the study had been allowed to continue and the harm from the higher hemoglobins had reached significance, whether the subsequent decade would have been different.
Following Besarab et al, we had Foley et al, Furuland et al, and Parfrey et al look at increased hemoglobins over 13 g/dL in dialysis patients and none of them was able to show improved mortality or cardiovascular outcomes. But the lack of science did little to slow the increasing doses and increasing average hemoglobins. As the science predicted, the increase in hemoglobin did not improve dialysis outcomes:

Data from USRDS.
The situation grew in 2006 with the publication of the updated KDOQI guidelines on anemia. These guidelines were underwritten by Amgen, the manufacturer of epoetin alfa, and 14 of the 16 committee members had received honoraria or grants from industry with financial ties to the guideline. The 2006 KDOQI guideline, in the face of the above data, actually increased the target hemoglobin from 11-12 to 11-13 g/dL. The old 11-12 target was first published in the 1998 KDOQI guideline and reaffirmed in the 2001 guidelines. When the 2006 guideline was published, the FDA label for EPO set the maximum hemoglobin at 12.
As the committee was finishing their guidelines, they received word about the soon-to-be-published CHOIR trial, but chose to maintain their methodology that limited themselves to only published, peer-reviewed literature. This decision set the stage for truly unfortunate optics. CHOIR found that higher hemoglobin targets resulted in a 33% higher rate of CV events and death. The CHOIR results were announced at the NKF Spring Clinical Meetings on April 20, 2006, and the revised KDOQI anemia guidelines, promoting higher hemoglobins based on opinion, were revealed at the same meeting the next day.
CHOIR, CREATE, TREAT and the end of normalization of hemoglobin.
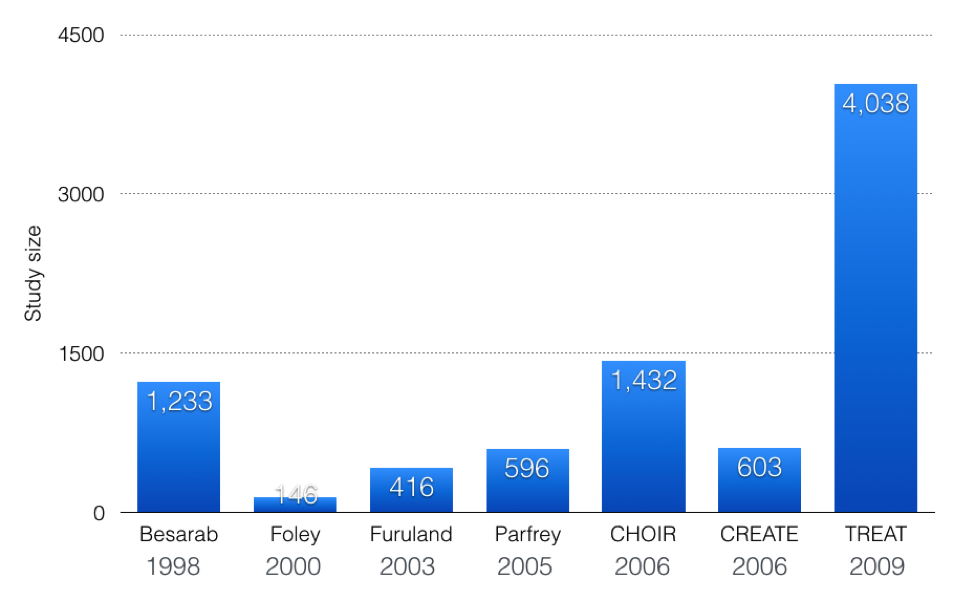
CHOIR and CREATE were two almost identical, open-label, studies that randomized pre-dialysis CKD patients to higher hemoglobins. CHOIR was larger, with 1,432 patients with eGFRs of 15 to 50 mL/min. The primary endpoint, a composite of death, myocardial infarction, hospitalization for heart failure, and stroke, was more likely with higher hemoglobins (HR 1.34; 95% CI, 1.03 to 1.74; P=0.03). There was no improvement in quality of life with the higher hemoglobin. CREATE had 603 patients with an eGFR of 15 to 35 ml/min. The primary outcome was time to first CV event, with no difference between groups. They did however find an increase in starting dialysis in the group with the higher hemoglobin, P=0.03.
The definitive word on high hemoglobins was the TREAT trial. This was a study of pre-dialysis CKD patients with diabetes. There are a few of unique features of TREAT that make it the strongest evidence we have on normalizing hemoglobin:
- It was big; TREAT had more patients than Besarab et al, CHOIR, and CREATE
- It was double blinded.
- It was placebo controlled. All the previous trials had active controls. In TREAT, patients received placebo injections; however, if their hemoglobin fell below 9, this was changed to darbepoetin to prevent (or decrease) transfusions.
The primary outcome was CV event or death. The primary outcome was negative. There was no advantage of darbepoetin; however, in subgroup analysis there was an increase in stroke with darbepoetin. There was 101 strokes with darbepoetin compared to 53 with placebo. There were fewer cardiac revascularizations and transfusions with the higher hemoglobin target.
The most amazing thing about the detour to hemoglobins of 13 and beyond is that we knew everything we needed to know from 1998’s Normalization of Hemoglobin trial. Then we spent the next 10 years trying to deny what was shown in that RCT. Much of the enthusiasm for high hemoglobins was derailed by CHOIR and CREATE, and then TREAT convinced the most important people of all, the group paying for this. In January of 2011, CMS added ESAs to the capitated dialysis bundle and in June the FDA amended the label of epoetin alfa and darbepoeitin to include some of the less-flattering study results and recommending against using it to raise the hemoglobin over 11. These changes caused ESA doses and hemoglobins to fall back to earth.
In February 2016, JASN posted the best postscript for this story. NephMadness alum Glenn Chertow and colleagues looked at the rates of a number of cardiovascular outcomes and found that in 2011 and 2012 (the years with the biggest fall in average hemoglobins), heart failure, stroke, and venous thrombosis were all lower than predicted. Taking our foot off the ESA accelerator seems to be benefiting patients.

Data from Chertow et al.
ACEi and ARB combination therapy for proteinuric kidney disease
 The high-impact and late-breaking clinical trials session at Kidney Week is one of the best sessions offered. It is guaranteed to offer novel data rather than rewarmed old data that you already discussed in journal club months ago. Regardless of whether the trial is positive or negative, the high-impact trial session always shines a flashlight on the dark underexplored corners of nephrology. In 2013, the highlight of the session was VA NEPHRON-D, a trial that looked at combining ACEi and ARB in diabetic kidney disease to reduce proteinuria and reduce the progression of CKD. There wasn’t too much suspense because the data safety monitoring board had stopped the study early due to a safety signal. The high-impact trial session opened the books on the negative trial.
The high-impact and late-breaking clinical trials session at Kidney Week is one of the best sessions offered. It is guaranteed to offer novel data rather than rewarmed old data that you already discussed in journal club months ago. Regardless of whether the trial is positive or negative, the high-impact trial session always shines a flashlight on the dark underexplored corners of nephrology. In 2013, the highlight of the session was VA NEPHRON-D, a trial that looked at combining ACEi and ARB in diabetic kidney disease to reduce proteinuria and reduce the progression of CKD. There wasn’t too much suspense because the data safety monitoring board had stopped the study early due to a safety signal. The high-impact trial session opened the books on the negative trial.

Data from Fried et al.
The results showed no slowing of CKD progression or improvement in mortality. This was largely confirmatory, as the theory that combined ACEi/ARB therapy had been on the ropes for a few years. Five years earlier, ONTARGET investigated the ACEi/ARB combination of ramipril + telmisartan against two controls: ramipril alone and telmisartan alone. No benefit was seen and there was increase in the composite outcome of doubling of serum creatinine, dialysis, and death with combination therapy (HR 1·09, 1·01–1·18, p=0.037). Though there are some good reasons that ON TARGET should not be extrapolated to typical CKD patients, it was difficult to take your eyes off the loss of GFR with dual therapy. Other studies of combined RAAS inhibition, like ASCEND (which added an endothelin antagonist to either an ACEi or ARB or both), found the desired reduction in proteinuria but additional adverse outcomes including CKD progression, fluid overload, and death.
So the end of the ACEi/ARB combination story comes, as the end often does, at the hands of a few well-done RCTs with appropriate endpoints. But how did the story begin? Why did nephrologists seek to double cover the RAAS with both ACEi and ARB?
Soon after the invention of ACEi, studies showed that in most patients it failed to suppress angiotensin 2 and aldosterone levels for more than a few hours. This was coined “ACE escape.” This phenomena was more a pharmacologic curiosity than a therapeutic target until 1995 when losartan, the first angiotensin receptor blocker, was introduced. This novel method of blocking the RAAS offered the possibility of dual blockade and elimination of ACE escape. Then in 1999, Russo et al published a study in AJKD showing a significant additive effect of combining ACEi and ARB to reduce proteinuria in non-nephrotic IgA nephropathy:

Data from Russo et al.
In this and in subsequent studies, the reduction in proteinuria was out of the proportion to reductions in blood pressure, which were typically modest (< 5 mm Hg) with the addition of the second agent. In a 2006 systematic review the average drop in proteinuria was 440 mg (less for diabetic disease, more in non-diabetics), with a reduction in blood pressure of 4.5/2.5 mm Hg. The average increase in potassium was only 0.11 mmol/L. With data like these, use of combination therapy looked like a no-brainer, a significant drop in proteinuria at the cost of a minimal drop in blood pressure and tiny increase in potassium. But the authors of the systematic review cautioned that little of the data was long-term and up to now we only had access to surrogate end points.
But what really lit the candle of dual blockade was COOPERATE. COOPERATE was published in The Lancet in 2003. It was a Japanese study of 263 patients with IgA nephropathy that were randomized to losartan, trandolapril, or a combination of both. After three years 23% of both the trandolapril and losartan alone groups had a doubling of serum creatinine or dialysis while only 11% of the dual therapy patients reached this end point. This was the missing link — the study that connected the great surrogate measures with improved patient outcomes. A 2011 WSJ article reported that by 2008, 140,000 patients in the US were being treated with dual therapy.
Unfortunately, in 2009 the study was retracted. The first concern was raised in 2008 by Regina Kunz who was examining the paper as part of a systematic review. She found numerous statistical improbabilities, sloppy methods, and an unrealistic (76%) reduction in proteinuria. This led to The Lancet prompting the hospital to initiate an investigation:
The hospital investigating committee examined medical records at another Japanese hospital where Dr. Nakao said he and his colleagues had done the research on 336 patients. But committee members “were not able to identify even a single patient who matched the contents of the paper,” said Yutuka Sanada, the president of the hospital that investigated, called Showa University Fujigaoka Hospital, in Yokohama. (from WSJ)
The 2009 retraction notice stated the following problems:
- Lack of IRB approval, despite claiming to have one
- No written patient consents
- No involvement of a statistician
- Failure to double blind the patients
- Unable to verify the authenticity of the data
COOPERATE now lives on as the seventh most-cited article to be retracted, with 572 citations before retraction and 101 after retraction!
Dual ACEi/ARB therapy captured the imagination because nephrologists have made proteinuria public enemy number one, but we are now living in an era where we have numerous examples of drugs or combinations of drugs that lower proteinuria without providing the expected improvements in long-term outcomes. This surrogate end point can no longer be trusted to predict hard outcomes. The lessons of combination therapy are two:
- don’t trust unvalidated surrogate outcomes
- be extra suspicious of papers that show results even better than you hoped to see
Aluminum Binders for Hyperphosphatemia vs Steroids for Idiopathic Membranous
Aluminum Binders for Hyperphosphatemia
 With the widespread availability of long-term dialysis in the 70’s, it was reasonable to wonder if new disease states would emerge due to the emergence of prolonged states of stable uremia. Would we see dialysis-induced diseases? With the discovery of dialysis encephalopathy in 1972 the answer was clearly “yes.” George Dunea described a patient with the syndrome:
With the widespread availability of long-term dialysis in the 70’s, it was reasonable to wonder if new disease states would emerge due to the emergence of prolonged states of stable uremia. Would we see dialysis-induced diseases? With the discovery of dialysis encephalopathy in 1972 the answer was clearly “yes.” George Dunea described a patient with the syndrome:
In the fall of 1972, one of our dialysis patients at Cook County Hospital in Chicago began to have episodes of bizarre behavior. During these episodes, she would stutter or become unable to speak, exhibit twitching movements, and have spells of confusion or altered consciousness, at times becoming almost comatose.
By 1976 nephrologists had tracked down its cause to aluminum intoxication from high levels of daluminum in dialysate. In addition to encephalopathy, osteomalacia and microcytic anemia were recognized consequences of aluminum toxicity. The young therapy of dialysis adapted and dialysate was regulated to have less than 10 µg/L of aluminum. Soon after the clusters of encephalopathy and osteomalacia disappeared only to be replaced by sporadic episodes of dialysis encephalopathy. Systematic aluminum toxicity was gone but the disease kept popping up. One possible source of aluminum was from the dominant phosphorus binder of the time, aluminum hydroxide. However, one problem existed: experiments had shown that GI absorption of aluminum was almost nonexistent.
Then in 1984 Dr Sharon Andreoli at Riley Hospital at Indiana University had an infant with advanced (but not dialysis dependent) CKD who developed respiratory distress due to multiple pathologic rib fractures. The child was on aluminum binders and Dr. Andreoli wondered if she could be having aluminum-induced osteomalacia from the oral aluminum load. A bone biopsy confirmed her suspicion. She then found 7 other children using aluminum binders for non-dialysis-dependent CKD. She ultimately showed the serum aluminum levels correlated with elemental aluminum dose in mg/kg/d (R2=0.81). She recommended minimizing the dose of aluminum and adding calcium carbonate to assist in controlling phosphorus. The aluminum load in infants at the time was quite high due to a diet that is largely milk based (today, there are low-phosphorus formulas that ameliorate this problem). Though Andreoli did not address adults on dialysis, the accompanying editorial speculated that adults with long slow exposures to aluminum could be suffering from the same problems Andreoli found in her patients.
1986 brought another advancement in the understanding of aluminum toxicity. Sodium citrate (Shohl’s solution) was sometimes added to aluminum hydroxide to treat metabolic acidosis. Patients on this combination developed acute, critical, and often fatal aluminum levels. Citrate increased aluminum absorption by opening tight junctions in the intestinal mucosa due to localized decreases in ionized calcium levels.
Unfortunately, the technology available to patients in the 80’s did not leave many good options. Calcium acetate had yet to be brought to market and hemodialysis was less efficient at clearing phosphorus than today’s dialysis. A 1986 review of aluminum toxicity saw no choices besides aluminum-based binders. The calcium- and magnesium-based binders of the time were not very effective and the dialysis itself was less efficient. Calcium carbonate is an inefficient binder so adequate control of phosphorus would take a tremendous number of pills and predispose the patient to hyperphosphatemia. In 1989 Taylor Swift was born and calcium acetate was shown to be twice as efficient as calcium carbonate for binding dietary phosphorus. However, the authors caution that even though calcium acetate is better than carbonate it is still relatively ineffective and patients will need to continue to use dietary discretion.

Data from Mai et al.
With the introduction and wide availability of calcium acetate, aluminum fell by the wayside. The 2003 KDOQI bone recommendations suggest no more than a single use of up to four weeks of aluminum only once in the life of a dialysis patient, and even then only for a phosphorus over 7 mg/dL.
This wrong turns reflects the limits of the knowledge and technology available. It used the best medicines and techniques available, yet emphasizes how everything we feed patients can accumulate and cause unanticipated side effects. And certainly the aluminum experience informs the current debate on calcium-based versus calcium-free binders. And the band plays on.
Steroids for Idiopathic Membranous
 Membranous nephropathy is undergoing a revolution with multiple therapies in use or being tested in certain populations.
Membranous nephropathy is undergoing a revolution with multiple therapies in use or being tested in certain populations.
- Italian Ponticelli protocol: chlorambucil alternating with methylprednisolone
- Modified Ponticelli protocol: oral cyclophosphamide alternating with methylprednisolone
- Dutch protocol: oral cyclophosphamide plus steroids with methylprednisolone
- Cyclosporine
- Tacrolimus
- Mycophenolate mofetil
- Rituximab
- ACTH
Vetting of these treatments is also advancing with the identification of the first major antigenic target in idiopathic membranous nephropathy: M-type phospholipase A2 receptor (PLA2R). This revolution in membranous comes after a prolonged period where the conventional treatment was oral steroids that have subsequently been shown to be no better than placebo.
The first use of steroids for nephrotic syndrome was done in 1950 by John Luetscher et al and published in Journal of Clinical Investigation. They found a dramatic response in half of their patients. The 1950 study assumed all cases of nephrotic syndrome were due to a single disease, and though they mentioned that some of their patients were children, there was no mention if the children were more likely to respond than not. In subsequent years, it became clear that children tended to have a brisk and complete response to steroids while adults had a less-predictable response. In 1962 a meeting of the British Medical Research Council on nephrotic syndrome recommended randomized controlled trials in adults to determine the effect of prednisone. The first trial was performed by Black et al and published in 1970. They enrolled all adults with nephrotic syndrome and then divided them into three groups by biopsy results:
- Group A with minimal change, 31 patients
- Group B with membranous nephropathy, 19 patients
- Group C with proliferative glomerulonephritis, 75 patients
Patients were randomized to placebo or prednisone. The dose was at least 20 mg/d and further titration was left up to the treating physician. A clear response was seen in Group A patients, but no treatment effect could be determined in groups B and C.
It was against this background that the Collaborative Study of Adult Idiopathic Nephrotic Syndrome was launched. It was an ambitious study that was going to be the largest and first placebo-controlled randomized trial of steroids in membranous nephropathy. Every patient enrolled had their biopsy read by a central pathology group. Patients were randomized to 125 mg of prednisone every other day for 8 weeks and then the dose was tapered according to response (slow for responders, quickly for non-responders). 154 people were biopsied for inclusion, of which 72 were enrolled. The researchers measured response to therapy on two axes, proteinuria and kidney function. Regarding proteinuria, steroids showed a transient therapeutic effect that disappeared; by the end of the study period, there was no difference between the two groups:

In regard to kidney function the results were more impressive:
- GFR fell 2 mL/min/y for steroid-treated patients versus
- GFR fell 10 mL/min/y for placebo-treated patients
- Serum creatinine doubled in 9 placebo patients, 7 within 12 months of randomization
- Serum creatinine doubled in 2 patients randomized to steroids
To understand the impact of this trial, it is useful to look at the words of Richard Glassock three years after it was published. In the review Glassock examined the gamut of observational and experimental data on the treatment of membranous nephropathy and had summarized the collaborative study thusly:
This study, rigorously designed and executed, represented the first and, up to that time, the only satisfactory controlled study of treatment of membranous glomerulopathy with corticosteroids and the results are, in my opinion, quite unequivocal. Namely, steroids do benefit the course of idiopathic membranous glomerulopathy.
The study was not entirely satisfying in that there was no difference in the proteinuria, so it was a bit difficult to understand how a therapy that didn’t change the fundamental symptom of membranous nephropathy could drive the dramatic difference in the GFR. Additionally the control group did disturbingly poor. Having 9 of 38 patients double their creatinine in 12 months is much worse than nearly any other other membranous cohort. The positive findings of the Collaborative Study were effectively being driven by an extremely unlucky cohort of patients who all landed in the placebo group. Additionally the results have a fragility index of 2 (i.e., if just two patients had better creatinine results in the placebo group the results would not have been significant.)
These concerns led to two additional and definitive studies, one from Canada that reported no response from steroids in 1989 and one from England that similarly showed no effect in 1990. These studies took a decade to plan and execute so that for ten years the highest-quality evidence on idiopathic membranous nephropathy was just wrong. A generation of nephrology fellows dutifully answered “alternate day steroids” on their board exams when asked how to treat membranous nephropathy. It might be impossible to determine but almost certainly, despite the reversal in 1989 and 1990, practitioners continued to offer the ineffective therapy, probably for a few years. It would be convenient if we could blame this misstep on fraud like with COOPERATE, or on drug companies promoting an expensive drug like with hemoglobin, or on a lack of alternative medications as with aluminum, but the membranous story is really just a story of how bad luck and long lead times can derail the truth for a decade or more. It should caution anybody from accepting a single RCT as gospel.
– Post written and edited by Dr. Joel Topf.





Spanish article describes 41 HD patients that received low dose Aluminum-based phosphate binder, 910 mg per day (463 g entire exposure) for a mean total time of 18 months. There were no signs of Aluminum toxicity (worsening anemia, decreased mean corpuscular volume, increased erythropoietin use, or lower PTH levels). They concluded that aluminum phosphate-binders were effective, economical and, with an apparent better security profile than in a previous time (Arenas et al., 2008).
http://www.revistanefrologia.com/en-publicacion-nefrologia-articulo-use-aluminum-based-phosphate-chelators-in-hemodialysis-in-era-ultrapure-water-X2013251408032908
Another study, 39 HD patients were studied. Mean dose of Aluminum was 1660 mg of dried aluminium hydroxide per day (equivalent to 609 mg of aluminium) over 23 months. They concluded that oral aluminum exposure was considerable. Yet no patients undergoing HD with RO treated water had evidence of Aluminum toxicity despite doses equivalent to 1660 mg of dried aluminium hydroxide per day for 2 years (Pepper et al., 2011).
http://bmcnephrol.biomedcentral.com/articles/10.1186/1471-2369-12-55