#NephMadness 2018: Women’s Health Region
Submit your picks! | NephMadness 2018 | #NephMadness | #WomensHealthRegion

Copyright: Gustavo Frazao / Shutterstock
“It is said you are never defeated unless you give up. We’re not going to give up.”
– Pat Summitt, the all-time winningest coach in NCAA basketball history.
Over a century ago, 100 women from seventeen different countries gathered to establish an annual day to promote equal rights for women. Since its inception, International Women’s Day has become an opportunity to celebrate the economic, political, social, and cultural achievements of women from around the world. This year, International Women’s Day and World Kidney Day are commemorated on the same day, March 8th. March is also National Kidney Month, which is when NephMadness begins! Given this amazing trifecta, we feel compelled to highlight critical yet underrecognized and understudied areas in women’s health as they pertain to kidney disease.
Selection Committee Member for the Women’s Health Region:
Dr. Hladunewich is an Associate Professor of Medicine at the University of Toronto in Toronto, Ontario, Canada. She is the medical lead for the Kidney Disease and Pregnancy Clinic (PreKid Clinic). Her research program includes studies in glomerular-based disease as well as pregnancy-related kidney disease.
Competitors for the Women’s Health Region
Reproductive Planning vs Menopause in CKD
Preeclampsia’s Global Impact vs Prematurity’s Global Impact
Reproductive Planning

Copyright: Andy Shell / Shutterstock
Reproductive health and preconception counseling are considered to be basic components in a woman’s trajectory of medical care, yet many women with kidney disease do not have these conversations with their nephrologists. Qualitative analyses have revealed that kidney patients have a strong desire to become mothers, suffer from intense grief after failed attempts at conception, lack information and awareness regarding birth defects, and fear exacerbation of their underlying disease.
In this section, we provide a brief overview of some key challenges and points of conversation in reproductive health that nephrologists must be aware of when caring for women with kidney disease.
Fertility
Fertility wanes as chronic kidney disease (CKD) progresses. Limited data exists regarding fertility in women with advanced CKD and end-stage kidney disease (ESKD). The few existing retrospective and observational studies suggest that rates are approximately one-fortieth of the general population in women with ESKD, though fertility appears to be slightly higher among women with greater residual kidney function.
With regards to the mechanism of this infertility, it is generally thought that as kidney function worsens, there is a proportional increase in hypothalamic pituitary gonadal (HPG) axis dysfunction. Women with kidney disease may experience amenorrhea due to lower levels of estrogen and progesterone. Additionally, the lack of a cyclical release of gonadotropin releasing hormone leads to a loss of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) pulsatility, resulting in anovulation. High levels of prolactin are also seen in kidney disease, thought to be related to the hormone’s increased pituitary production and decreased renal secretion.

Ahmed SB, Semin Nephrol
Not only must nephrologists be aware of the decreased fertility in their patients, but they should be cognizant of certain medications’ effect on reproductive health. When caring for women with kidney disease who desire pregnancy and need cyclophosphamide treatment, the agent’s links to premature ovarian failure must be discussed. Studies have shown that this risk increases with advancing age and greater cumulative dose. In women who desire to retain fertility, regimens that utilize a lower cumulative dose of cyclophosphamide should be considered, in addition to co-treatment with gonadotropin-releasing hormone agonists. With the availability of mycophenolate mofetil and rituximab, alternative treatment options should also be contemplated where appropriate.
Because women who receive a kidney transplant have improved fertility, decreased sexual dysfunction, and fewer fetal risks, nephrologists must review options for transplantation as a part of reproductive counseling.

Pregnancy-associated risks and management challenges
Pregnancy in the setting of kidney disease is risky at any stage, and despite improvements in prenatal care and outcomes, women must be counseled regarding the potential for adverse events. Though the chance for developing preeclampsia is greater in those with more severe kidney disease, even women with milder kidney disease are at risk. Women with CKD also have increased rates of preterm labor, intrauterine growth restriction, neonatal death, maternal mortality, and gestational hypertension. Further, those with lower estimated glomerular filtration rates (eGFRs) of less than 40 mL/min/1.73m2 and proteinuria greater than 1 g/day are more likely to have a faster rate of decline of GFR post-delivery.
Though pregnancy in the setting of ESKD has become safer and mean gestational age at delivery has increased from 30.5 to 33.8 weeks, these remain complex pregnancies to manage. Women with ESKD who do become pregnant also need to be prepared for more intensive dialysis regimens than those to which they may have previously been accustomed. Current recommendations for dialysis in pregnancy include dialyzing patients for a minimum of 36 hours per week in the absence of residual kidney function, targeting a near-normal pre-dialysis blood urea concentration, and aiming for a post-dialysis blood pressure of <140/90 mm Hg. As water-soluble vitamins and minerals are lost during dialysis, some experts recommend doubling the daily dose of multivitamin given to pregnant patients. Protein and caloric intake should be closely monitored, and given the role of calcium in fetal development, the dialysate calcium concentration may need to be increased. Additionally, patients may need to be prepared for increased erythropoietin and iron requirements to avoid the need for transfusions.
With regards to transplant recipients, current guidelines suggest that conception should not jeopardize graft survival one year post-transplant under the following circumstances: the patient has had no rejection within the past year, has adequate allograft function with a creatinine level of 1.5 mg/dL or less, has minimal proteinuria, has not experienced infections that could impact the fetus such as cytomegalovirus, and has maintained immunosuppression at therapeutic levels. However, pregnancy-associated risks persist in these patients as well. Pregnant kidney transplant recipients are at a higher risk for preterm delivery, intrauterine growth restriction, graft loss, rejection, and up to 30% experience preeclampsia. In addition, it is important to explain to patients that all immunosuppressants cross the maternal-fetal barrier and that anti-rejection medications may need to be adjusted. Most immunosuppressants have been labeled by the Federal Drug Agency as either Pregnancy Category C or D, and we have limited data regarding their long-term fetal impact.
Contraceptive counseling
Given that fetal and maternal survival is much worse than in age-matched controls, kidney patients who do not wish to become pregnant need active contraception counseling. Unfortunately, studies show that nephrologists rarely discuss contraception.
No specific methods of contraception are considered safer than others, and the choice of agent depends on patient preference and comorbidities. Absolute contraindications to estrogen-based oral contraceptive pills include severe cardiovascular disease, a history of venous thromboembolism, concurrent breast cancer, current smoking in a woman over 35, and impaired liver function. Relative contraindications include systemic lupus erythematosus, hypertension, hypertriglyceridemia, and diabetes mellitus. Barrier methods, intrauterine devices (IUDs), and tubal ligation have similar failure rates as the general population and are all viable options for contraception in this patient population.
Conclusion
Many unknowns persist with regards to reproductive counseling in kidney patients. We have limited disease specific outcome data. No data exist on assisted reproductive technologies in this patient population. We have limited information about pregnancy rates and complications among women on peritoneal dialysis. We also have little knowledge of the long-term outcomes of children who were exposed to immunosuppressant medications in utero or who were born to a mother on dialysis.
Despite these and many more uncertainties, vast improvements in prenatal care have made successful pregnancies in the setting of kidney disease much more common. For this reason, we as nephrologists must not shy away from broaching these conversations with our female patients who may be interested in starting families. By working within an interdisciplinary care team of high-risk obstetricians, social workers, nurses, and health psychologists, we must help our patients make informed decisions about their reproductive care that are in line with their personal goals.
Menopause and CKD

Copyright: Monkey Business Images / Shutterstock
Women are more likely to have CKD than men (although they are less likely to develop ESKD), and our kidney population continues to age. Despite this, hormonal dysregulation and menopause are aspects of women’s health that are under-recognized by the nephrology community and rarely discussed with patients. A survey of 175 nephrologists from Canada, Australia, New Zealand, and the United Kingdom revealed that 35% discussed fertility issues and only 15% broached the topic of menstrual irregularities. Forty-three percent of the physicians surveyed cited that this was due to an uncertainty regarding the available literature as well as a lack of information regarding post-menopausal hormone replacement therapy.
Though it is known that women across all stages of CKD suffer from accelerated aging phenotype, excessive bone fractures, and premature cardiovascular events, little is known with regards to these patients’ reproductive hormones and menopausal characteristics. The mechanism for earlier menopause in CKD remains unknown. Much of what is currently known regarding menopause in kidney disease has been nicely summarized in Ahmed’s review of the topic, which provided a formative basis for our overview below.
In the United States, women reach menopause on average by the age of 51. This occurs earlier in the setting of both ESKD and CKD. A study of over 17,000 post-menopausal women showed that women with CKD were more likely to have experienced menopause before the age of 45 as compared to those with normal kidney function. Primary ovarian insufficiency, or the cessation of menses prior to the age of 40, is also more commonly seen in patients with CKD.
Despite its earlier onset, menopause can be challenging to diagnose in patients with kidney disease. The use of recombinant erythropoietin has resulted in a decreased frequency of amenorrhea in women with ESKD, and switching from conventional home dialysis to a nocturnal modality has been shown to affect menstrual cycles and estradiol levels. Though normal menses are often restored after transplantation, up to 31% of women with ESKD do not resume normal menses post-transplantation, and many women with normal menstrual cycles prior to transplantation develop amenorrhea afterwards.
Not only is little known regarding the pathophysiology of menopause in kidney disease, even less is known with regards to treating its sequelae. We do know that earlier menopause equates to an earlier onset of complications associated with poor bone health, such as osteoporosis. Once a postmenopausal woman with kidney disease is diagnosed with osteoporosis, the decision to initiate pharmacologic therapy is based on her history of fragility fracture and absence of concurrent renal osteodystrophy. Therapy is generally initiated as long a a patient’s GFR is > 30 mL/min. Those with more severe kidney disease may require further evaluation with a bone biopsy or further biochemical testing to rule out concurrent metabolic bone disease prior to treatment.
Post-hoc analyses of trials that have included post-menopausal women with moderate-to-severe CKD have demonstrated the safety and efficacy of oral formulations of bisphosphonates such as alendronate and risendronate. Given the lack of robust data associated with raloxifene and the hypocalcemia shown with denosumab use, these therapies tend to be utilized less frequently. Many nephrologists also avoid bisphosphonates in the setting of a GFR <30 mL/min, due to the drugs’ renal clearance and association with kidney failure when given intravenously.
The role of postmenopausal hormone therapy (HT) has re-emerged as an area of research that has garnered interest based on the premise that HT may play a protective role in cardiovascular risk. A recent meta-analysis using oral medroxyprogesterone, oral or transdermal estradiol, and (in some cases) oral raloxifene revealed a slight improvement in patients’ lipid profiles with minimal side effects. However, effects on cardiovascular outcomes were not examined.
Observational studies among dialysis patients have highlighted improvements in menstrual function, a decrease in prolactin level, and an decrease in hip and spine bone mineral density after HT therapy. Larger, well-designed trials of HT in kidney patients, including studies that focus on specific cardiovascular outcomes and alterations in kidney function, are warranted.
It is important for nephrologists to be aware of the clinical implications of early menopause in aging female patients. Not only does early menopause have harmful effects on cardiovascular risk and mineral bone health, but it also has the ability to influence our patients’ quality of life. Increasing our awareness of knowledge gaps in this area is critical to facilitate formative research and to have constructive conversations with our patients about this increasingly relevant aspect of their kidney care.
Preeclampsia’s Global Impact

Copyright: didesign021 / Shutterstock
Preeclampsia is defined as new onset hypertension accompanied by either new onset of proteinuria or end organ dysfunction such as thrombocytopenia, liver dysfunction, new CKD, pulmonary edema, or new onset cerebral or visual disturbances that typically occur after 20 weeks gestation. Some consider it to be the most common glomerular disease worldwide.
While isolated hypertension occurs in approximately 10% of pregnancies with outcomes similar to normotensive pregnancy, preeclampsia occurs in 4-8% of pregnancies and is associated with significant morbidity and mortality. Hypertensive disorders are the second leading cause of maternal mortality (after embolism) in the United States and worldwide (accounting for 15% of maternal mortality in the US, 9% in Africa and Asia, and 26% in Latin America and the Caribbean).
Mortality rates are highest in low- and middle-income countries with limited access to emergency maternity services and neonatal intensive care. The rate of preeclampsia is increasing in the U.S., although the rates of eclampsia appear to be declining due to the treatment of preeclampsia with magnesium. With the rates of preeclampsia increasing we are seeing an increase in maternal mortality in the U.S..
To really understand the true burden of this disease we give you the following comparison: Given that there are over 200 million pregnancies worldwide annually, at least 8 million pregnancies are affected each year by preeclampsia. For comparison, it is estimated that IgA nephropathy develops in approximately 2.5 of every 100,000 people each year, and thus the annual incidence worldwide is approximately 190,000 cases per year.
Preeclampsia is a systemic syndrome characterized by vasospasm, activation of the coagulation system, and perturbations in volume and blood pressure regulation. It typically develops in the second half of pregnancy and while the exact cause is not known, the placenta may be the pathogenic focus, given that delivery is the definitive cure.
Several pathogenic mechanisms for development of preeclampsia have been proposed. Early in gestation, during what has been referred to as the first stage of preeclampsia, terminal branches of the uterine artery are thickened and transformed to eventually accommodate the growing volume of uterine blood flow. Failure of cells to remodel and express proper adhesion molecules may lead to placental hypoxia. Additionally, placental tissue in pregnancies affected by preeclampsia may express altered leukocyte antigen genes and inflammatory cytokines, leading to impaired maternal tolerance to the placenta, a process similar to transplant allograft rejection.
The so-called second stage of preeclampsia involves maternal systemic manifestations including inflammatory, metabolic, and thrombotic responses attempting to alter vascular function, leading to multi-organ involvement, primarily ischemic in nature. The primary pathologic finding on biopsy of a kidney affected by pre-eclampsia is glomerular endotheliosis, with enlarged and swollen, but not hypercellular, glomeruli. Intracapilllary cell swelling encroaches on the capillary lumen and the glomeruli appear bloodless under the microscope.
Functionally, glomerular filtration rate decreases, as does renal blood flow, and both can lead to acute tubular or cortical necrosis. Disruption of the endothelial glycocalyx is hypothesized to disrupt local symbiosis between endothelial cells and podocytes, leading to non-selective proteinuria. This allows proteins of various sizes to leak through the glomerulus, including larger proteins such as immunoglobulins.
Systemically, preeclampsia is thought to cause maternal hypertension secondary to increased peripheral vascular resistance due to hyperresponsiveness to angiotensin II (rather than the typical nitric-oxide mediated decrease in responsiveness to vasoconstrictors typically seen in pregnancy). Other systemic manifestations of preeclampsia include thrombocytopenia, disseminated intravascular coagulation, liver ischemia, and hemorrhage (leading to jaundice, HELLP syndrome, or hepatic rupture), and central nervous system manifestations (seizures, cerebral hemorrhage or edema, retinal edema, or blindness).
Given significant multi-organ involvement, it is not surprising that preeclampsia is associated with poor outcomes and can be life-threatening to the mother and fetus. Poor fetal growth, intrauterine growth restriction, preterm birth, placental infarction, placental abruption, or even fetal death can occur.
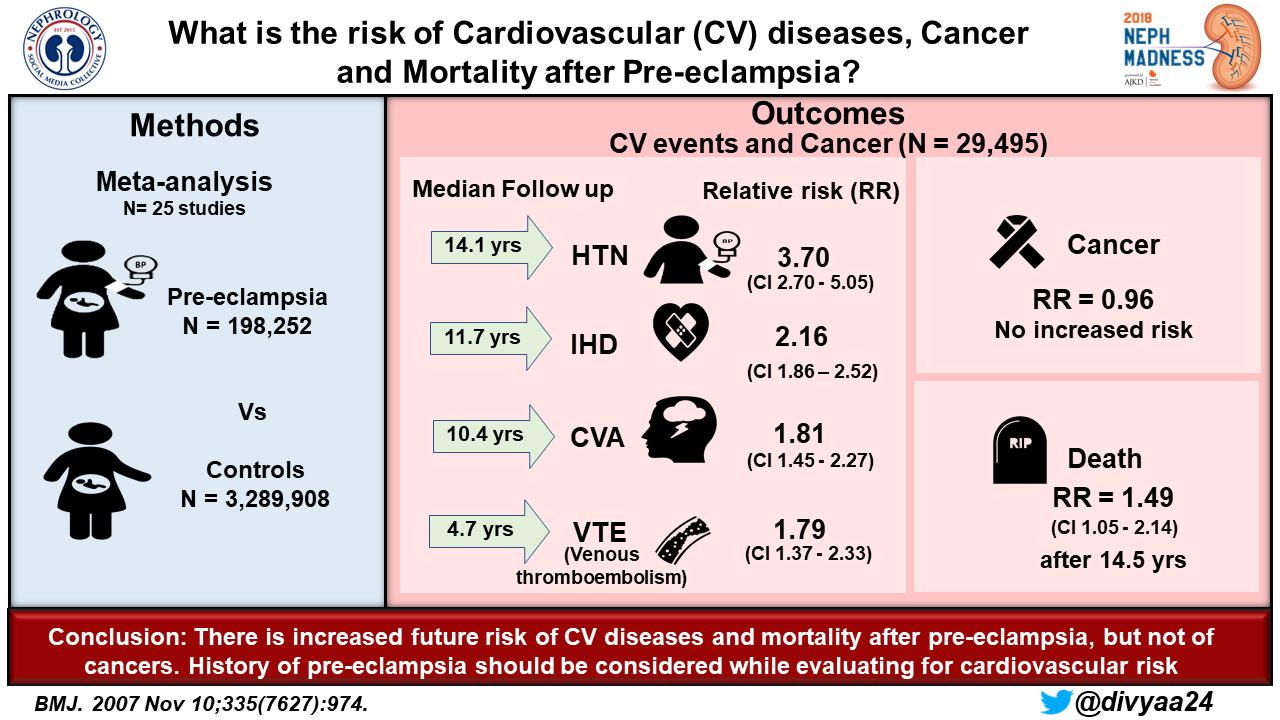
Preeclampsia has been associated with long-term complications, especially of the cardiovascular system and kidneys. In fact, the worse the pregnancy outcome from preeclampsia, the higher the risk of early cardiovascular disease. CKD is a risk factor for development of preeclampsia. Additionally, patients with preeclampsia without known kidney disease are at increased risk of developing kidney disease later in life, earlier than normotensive women, though it is not known whether preeclampsia has deleterious effects on the kidney or if there are baseline factors that predispose to both conditions.
A meta-analysis found that women with a history of preeclampsia have a risk of microalbuminuria similar to published prevalence in patients with type 1 diabetes mellitus. Even when controlling for confounders (including other comorbidities including hypertension, diabetes, or rheumatic disease), the relative risk of developing ESKD was 4.7 (for preeclampsia during the first of multiple pregnancies), 6.7 (for preeclampsia during the second pregnancy) or 6.4 (for pre-eclampsia during both pregnancies).
Having a low birthweight or preterm infant increased the relative risk of ESKD. Long-term complications of the vascular system have also been shown, including increased relative risk for hypertension (3.7), ischemic heart disease (2.2), stroke (1.8), and venous thromboembolism (1.8) after 4-14 years. Overall mortality is also increased (with a hazard ratio of 1.5 after 14.5 years).
Protecting the short- and long-term health of pregnant women should always be a priority in society, and thus special attention and resources should be given worldwide to preventing, identifying, and treating preeclampsia. As the most common glomerular disease worldwide, with high morbidity and mortality and potential lifelong complications, it is imperative for nephrologists to be familiar with pathophysiology and potential complications of this formidable condition. When investigating the source of CKD, nephrologists should consider pregnancy history to probe for a history of preeclampsia. Some women may not know if they had preeclampsia, and in this situation, low birth weight and preterm birth are good surrogate markers!
Prematurity’s Global Impact

Photo: OndroM / 123RF
Our patients with CKD, ESKD, and those who are post-kidney transplantation are all at higher risk of preeclampsia. Preeclampsia increases the risk of an infant being born prematurely. In addition, our patients have other factors that increase their risk of preterm birth in pregnancy, including an increased risk for premature rupture of the membranes and intrauterine growth restriction. Thus, it is important for us as nephrologists to educate our patients about the risks of preterm birth, which include both renal and nonrenal issues.
Preterm birth is not isolated to women with kidney disease. Globally, an estimated 15 million babies are born preterm, and preterm birth complications account for approximately 1 million neonatal deaths annually. Preterm birth complications are the most common cause of death for children under the age of five. Globally, 11.1% of births are estimated to be preterm, and the highest preterm birth rates occur in the lowest income countries. These countries demonstrate the worst infant survival rates, as half of the infants born at or before 32 weeks fail to survive.
Kidney development in neonates continues until 34-36 weeks gestation, as more than 60% of nephrons are formed in the last trimester of pregnancy. Kidney development does not occur postnatally except in the setting of infants who are severely preterm. In addition, organogenesis can be impaired antenatally due to factors such as intrauterine growth restriction. The combination of these factors is thought to explain why infants with low birth weight and infants born at an earlier gestational age have reduced nephron endowment. Brenner et al first hypothesized in 1988 that this reduced nephron endowment or “low nephron number” acquired in utero may be why certain populations have an increased risk of hypertension and CKD.
Preterm births are associated with several complications post-delivery. One cohort revealed that 18% of very low birth weight infants went on to develop acute kidney injury (AKI). Mortality rates among infants with AKI as opposed to infants with normal kidney function were 42% and 5%, respectively. Preterm infants are also at risk for “tubulopathy of prematurity,” a transient condition in which the concentrating ability of the kidneys is impaired, leading to a loss of electrolytes, bicarbonate, and small proteins. Sodium depletion leads to impaired postnatal growth, as does metabolic acidosis.
In the long run, low birth weight and prematurity offer the strongest correlation with low nephron number and are often used as surrogate markers in studies. Very low birth weight and prematurity appear to be risk factors for development of secondary FSGS. In addition, low birth weight has been linked to an increased risk for CKD later in life; a meta-analysis found a 70% increase in the relative risk of developing CKD! Both low birth weight and low nephron number have been associated with an increased risk of hypertension in some populations, including Caucasians and Australian Aborigines.
Preterm birth does not affect the kidneys in isolation, but its list of effects on other organ systems is long. Preterm infants are at an increased short-term risk of complications such as the following: respiratory distress, retinopathy, bronchopulmonary dysplasia, patent ductus arteriosus, necrotizing enterocolitis, and intraventricular hemorrhage. In the long term, risks of being born prematurely include frequent hospitalizations, cognitive abnormalities, motor deficits, cerebral palsy, vision losses, hearing losses, attention-deficit hyperactivity disorder, depression, generalized anxiety disorder, asthma, growth restriction, and insulin resistance.
As preterm birth is associated with both short-term and long-term morbidity and high mortality, education about its risks and outcomes should be a requisite part of pre-pregnancy counseling in our kidney patients. It is also an important risk factor that nephrologists should elicit when trying to determine a new patient’s cause of CKD, and nephrologists should be including a birth history in their initial assessment!
– Post written by Anna Burgner (@anna_burgner), Devika Nair (@devimol), and Diana Mina (@DiMiRenalMD)
How to Claim CME
US-based physicians can earn 1.0 CME credit for reading this region. Please register/log in at the NKF PERC portal. Click on “Continue,” click on the “Women’s Health Region,” then click on “Continue” to access the evaluation. You’ll need to click on “Continue” again to complete the evaluation, after which you can claim 1.0 credit and print your certificate. The CME activity will expire on June 15th, 2018.
Submit your picks! | NephMadness 2018 | #NephMadness | #WomensHealthRegion




Leave a Reply