Preparing to Fail: A Useful Tool for Predicting Time to ESRD in Pediatric CKD
Children with chronic kidney disease (CKD) face a heightened risk for progression and development of comorbidities due to their longer life expectancy from a young age compared to adults who develop CKD. Many pediatric centers have adopted the KDIGO classification for staging CKD, which estimates progression risk by cause, GFR category, and albuminuria category. However, this staging system was derived from adult data and the causes of CKD in children are very different from adults. According to 2014 data from the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS), congenital anomalies of the kidneys and urinary tract (CAKUT) are the most common cause of pediatric CKD requiring renal transplantation (36.2%), followed by acquired glomerular disorders including focal segmental glomerulosclerosis (11.7%) and chronic glomerulonephritis (3.1%). Though diabetes mellitus is the most common cause of CKD in adults, only 0.1% of children in the NAPRTCS report who received renal transplantation had diabetic kidney disease.
One of the major challenges that pediatric nephrologists face is how to counsel patients and their families on the timing of CKD progression. Will the family of a child with newly diagnosed renal dysplasia need to think about looking for living kidney donors within the next 3-5 years, or at all during the patient’s childhood? How does that change if the child has a different etiology for their CKD, such as a glomerular disorder, or can the time course be altered if proteinuria – a leading risk factor for CKD progression – is adequately managed?
Furth et al explored these questions by creating a modified KDIGO classification system for pediatric CKD, based on pooled data from the CKiD cohort and the ESCAPE trial. Using baseline GFR, degree of proteinuria, and etiology of CKD (broadly defined as non-glomerular vs glomerular), the group developed a progression continuum of six risk stages to predict the timing until a composite endpoint (- 50% GFR decline from baseline, GFR less than 15 mL/min/1.73 m2, or initiation of renal replacement therapy) was met. Akin to the NAPRTCS data, the most common non-glomerular diagnoses were CAKUT and the most common glomerular diagnosis was focal segmental glomerulosclerosis.
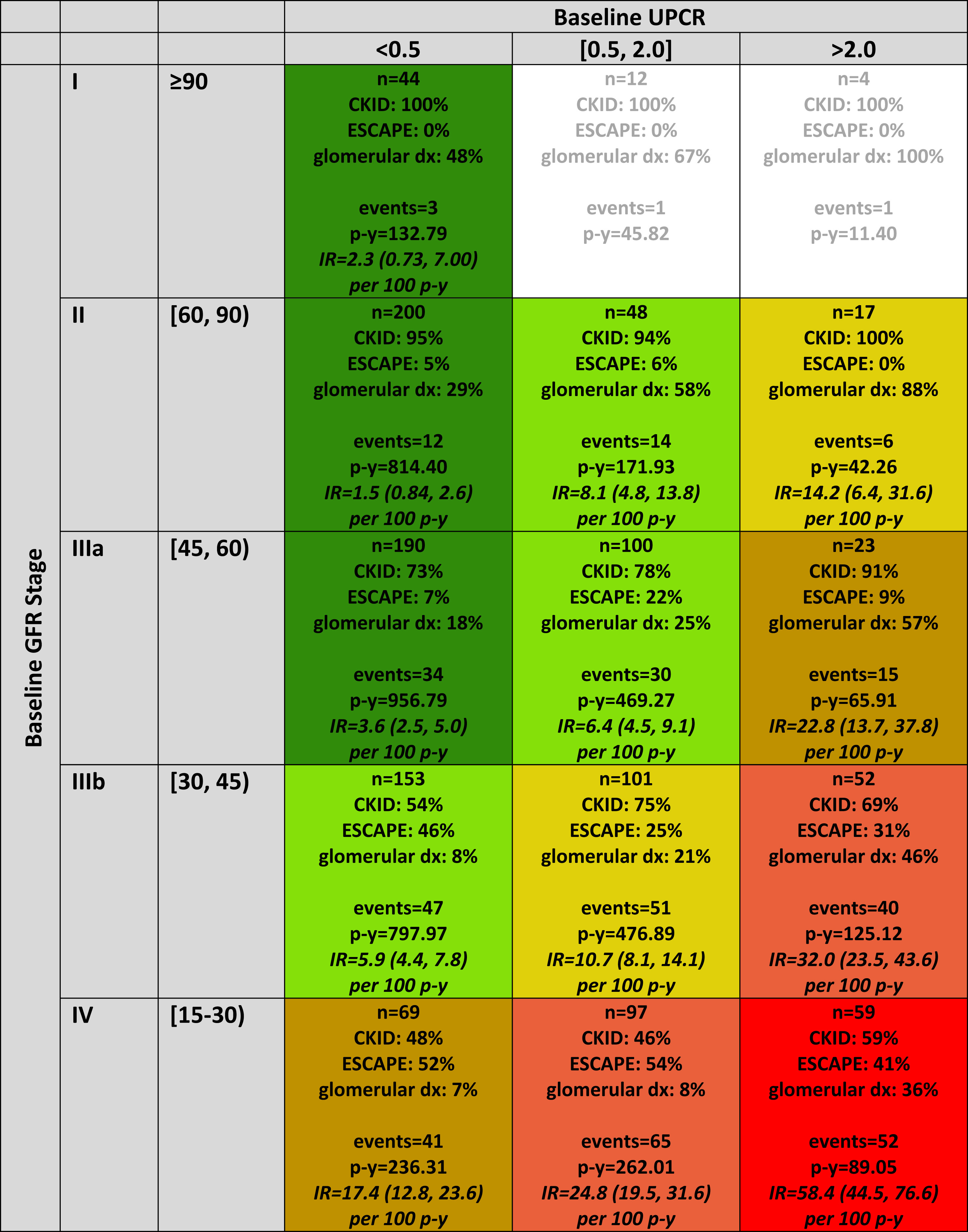
Distribution of persons, person-years, chronic kidney disease (CKD) diagnosis (dx), and events by categories of baseline glomerular filtration rate (GFR) and urine protein-creatinine ratio (UPCR), N = 1,169. Each cell in the table describes the number of participants, overall and distributed by cohort affiliation; prevalence of glomerular CKD; number of events; number of person-years (p-y); and empirical incidence rate (IR; per 100 person-years) of the composite outcome event (50% GFR decline, renal replacement therapy, or GFR < 15 mL/min/1.73 m2) with 95% confidence interval. Cell coloring defines the final 6 GFR/proteinuria risk stages ordered from best to worst prognosis as follows: A (dark green), B (light green), C (gold), D (tan), E (salmon), and F (red). IRs expressed as events per 100 person-years. Cells with fewer than 15 participants do not have an IR calculated. Figure 1 from Furth et al, AJKD © National Kidney Foundation.
Over a median follow-up of 3.8 years, the children most likely to reach the composite endpoint were those with a lower baseline GFR and a high degree of proteinuria (urine protein-to-creatinine > 2.0 mg/mg) regardless of baseline GFR stage. Having a glomerular disorder at any risk stage shortened the time to reach the composite endpoint by 43%. These findings are not surprising, but they provide perspective on the utility of using anti-proteinuric therapies to slow CKD progression and can help provide anticipatory guidance for when to start the pre-transplant evaluation in a child. Furth et al used an accelerated failure time model to predict, based on each risk group, the expected time in years to reach the composite endpoint. This may serve as a convenient reference for use in clinic when providing families anticipatory guidance.

Expected times, 10th, 25th, and 50th (percentile), from glomerular filtration rate (GFR)/proteinuria risk characterization (study baseline) to clinical composite event (50% GFR decline, renal replacement therapy [RRT], or GFR < 15 mL/min/1.73 m2), for 6 GFR/proteinuria risk stages, by chronic kidney disease (CKD) diagnosis. Estimated event times are generated from an accelerated failure time model using a conventional generalized gamma distribution with 7 beta indicator parameters: 6 risk stages (A-F) and glomerular CKD. Time to event is from baseline (time at which risk level was defined). Event is defined as RRT, GFR < 15 mL/min/1.73 m2, or 50% GFR decline. Figure 4 from Furth et al, AJKD © National Kidney Foundation.
By using this 6-stage risk model from a large data set of children with CKD, a pediatric center may better plan for when a child with CKD may need to undergo renal transplantation evaluation or consider dialysis options. Management of CKD in children is complex and multidisciplinary, and we need as much time as possible to provide the best care for our patients. In a world full of uncertainties, this study provides a useful tool for pediatric nephrologists to provide anticipatory guidance to patients and their families.
– Post prepared by Brian Stotter, AJKDBlog Guest Contributor. Dr. Stotter has a blog called Ins and Outs. Follow him @StotterMD.
A PDF of both figures can be downloaded here (FREE). To view the article by Furth et al (subscription required), please visit AJKD.org.
Title: Estimating Time to ESRD in Children With CKD
Authors: S.L. Furth, C. Pierce, W.F. Hui, C.A. White, C.S. Wong, F. Schaefer, E. Wühl, A.G. Abraham, and B.A. Warady, on behalf of the CKiD, ESCAPE Study Investigators
DOI: 10.1053/j.ajkd.2017.12.011

Leave a Reply