#NephMadness 2021: COVID-19 Region
Submit your picks! | NephMadness 2021 | #NephMadness | #COVID19Region
Selection Committee Member: Maria Jose Soler @PepaSolerR
Maria José Soler combines her role as an attendant Nephrologist at the Nephrology Department at Vall d´Hebron Hospital and an active role as a clinical researcher within the Experimental Nephrologist Group. In the COVID-19 pandemic period she has become part of NephJC’s Coronavirus Conundrum: ACE2 and Hypertension edition, and she is leader of the Working Group on RAAS in COVID-19 in the COVID-19 Vall d´Hebron Task Force.
Writer: Debbie Chen @dc_chen
Debbie Chen is a nephrology fellow at the University of California, San Francisco. Her research interests include pharmacoepidemiology, clinical decision-making, and individualization of medical therapies, and cardiovascular outcomes among patients with chronic kidney disease. She is a current AJKD Editorial Intern.
Writer: Christin Giordano @CGiordano1225
Christin Giordano is a second-year nephrology fellow at Vanderbilt University Medical Center (VUMC) and will be starting a career in private practice upon graduation. She completed her Bachelor’s degree at Brown University, medical degree from the University of Central Florida, and her internal medicine residency at VUMC.
Competitors for the COVID-19 Region
COVID-19 in Dialysis vs COVID-19 in Transplant
Glomerular Injury in COVID-19 vs Tubular Injury in COVID-19

Copyright: creativeneko / Shutterstock
COVID-19 in Dialysis vs COVID-19 in Transplant
COVID-19 in Dialysis

Copyright: mailsonpignata / Shutterstock
Hemodialysis centers represent a high risk location for transmission of COVID-19. Hemodialysis patients with COVID-19 are also at high risk for worse outcomes. The pandemic has continued to bring awareness to the need to expand home dialysis modalities!
Coronavirus infectious disease 2019 (COVID-19) has changed the daily lives of people around the world. Over 700,000 people in the United States (US) live with end-stage kidney disease (kidney failure) and are particularly vulnerable during this global pandemic. Patients with kidney failure are at high risk for exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and adverse outcomes related to infection. Here, we review the impact that COVID-19 has had on patients receiving dialysis, initiatives to prevent the spread of COVID-19, and clinical outcomes among this population.
In-center and home dialysis
In the United States, dialysis is delivered predominantly in-center. In the midst of shelter-in-place orders, patients requiring hemodialysis must leave their homes multiple times a week, transport to dialysis units, often through shared or public transportation, interact with staff and a number of patients, and receive dialysis treatment for several hours, often without the ability to properly socially distance. This creates unique challenges for the delivery of care for patients requiring hemodialysis.
In an early retrospective analysis from Wuhan, China, investigators found that hemodialysis centers were high-risk settings for SARS-CoV-2 infection and described interventions that effectively prevented transmission, including requiring patients to wear masks during dialysis, conducting universal screening, and isolating infected patients. A study of a large dialysis center in the United Kingdom found that within 6 weeks of the first detected case of COVID-19, over 20% of their patients on dialysis were infected. Dialysis centers around the world have been rapidly implementing strategies to minimize the risk of transmission of COVID-19. Well-established cleaning processes are part of the usual hemodialysis unit protocol, so transmission has been predominantly occurring within dialysis shifts rather than from one shift to the next. Universal use of simple surgical masks by patients and staff during dialysis procedures may be the most effective measure. The American Society of Nephrology (ASN), European Renal Association-European Dialysis and Transplant Association (ERA-EDTA), and Centers for Disease Control and Prevention (CDC) have established a COVID-19 response team that has offered official protocols to aid hemodialysis facilities in mitigating the burden of COVID-19 among hemodialysis patients. These experts have recommended universal face masks, social distancing of patients in waiting areas, protocols for early detection and isolation of infected patients, and the use of personal protective equipment for all health care providers.
It has been speculated that universal testing of all patients who receive in-center dialysis could mitigate transmission. One dialysis unit in Wuhan, China reported screening all patients and staff with chest computed tomography, but this is an impractical approach. While screening based on symptoms is recommended by the CDC, asymptomatic viral transmission of COVID-19 is well-recognized. One study performed in the United Kingdom revealed 36% seroprevalence of SARS-CoV-2, with 40% of cases found in asymptomatic patients. These patients, particularly the asymptomatic ones, may have been a source of infection transmission at their dialysis centers, highlighting the importance of the use of personal protective equipment in units. Another study in Spain revealed 19% SARS-CoV-2 seropositivity among patients receiving hemodialysis and found that 26% of those who tested positive remained asymptomatic after a 3-week follow-up. In the midst of the COVID-19 pandemic, home-based dialysis may be a useful way to reduce infection risk among patients with kidney failure. COVID-19 infection has been shown to be substantially less likely to occur in patients receiving dialysis at home compared to in-center. Patients receiving home dialysis also have the advantage of being able to use telehealth to communicate with their providers. Even before COVID-19, two kidney failure health care reform initiatives in the United States have increased support for home-based dialysis. The COVID-19 pandemic has further highlighted the importance of these initiatives.
COVID-19 therapeutic considerations in kidney failure
Dexamethasone was found to reduce mortality in patients requiring supplemental oxygen or mechanical ventilation in the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial. Dexamethasone is primarily metabolized hepatically and there are no dose adjustments required in patients with kidney failure receiving dialysis. Remdesivir, a nucleotide analog that inhibits viral RNA-dependent RNA polymerase, is approved by the US Food and Drug Administration (FDA) for use in hospitalized adults and children ≥ 12 years with COVID-19. Remdesivir was originally developed as an investigative drug for Ebola, but has been shown to reduce median time to recovery in hospitalized patients. However, patients with kidney failure were excluded from the clinical trials assessing the efficacy of remdesivir in COVID-19. In fact, most trials had estimated glomerular filtration rate (eGFR) cutoffs between 30 and 50 mL/min/1.73m2. This has been of notable concern given remdesivir is predominantly (74%) cleared by the kidney. Remdesivir’s carrier, sulfobutylether-β-cyclodextrin (SBECD), is also excreted through the kidneys, and its accumulation has been associated with nephrotoxicity. While there is currently insufficient data to indicate the safety of remdesivir among patients with eGFR < 30 mL/min/1.73m2, the limited duration of treatment (5-10 days), low concentrations of SBECD carrier, and dialyzability of remdesivir suggest that the risks of this therapy in patients with kidney failure may be relatively low.
Outcomes after COVID-19

Visual abstract by Pablo Garcia and Samira Farouk on Weiss et al.
The spectrum of outcomes after COVID-19 ranges from asymptomatic infection to fatal multiorgan failure. Those experiencing more severe symptoms are of older age and have more comorbid conditions such as hypertension, diabetes, and cardiovascular and pulmonary disease. A retrospective analysis of 59 patients in New York found a 31% overall mortality among patients with kidney failure and COVID-19. The authors note that many of the patients with kidney failure had expressed wishes against intubation but also note that mortality was 75% among those who received mechanical ventilation. Another study from New York of 2,178 patients on dialysis found a 14% prevalence of COVID-19 compared to 2.6% in the general New York City population at the time. Among the patients who were COVID-19 positive in this population, the mortality rate was 28%. Mortality was associated with age greater than 65 years and longer duration of kidney failure (a 10% increase for each additional year on dialysis). Notably, this study also revealed that immigration status (reflected by availability of Social Security numbers) was significantly associated with mortality. Another study using the OpenSAFELY health analytics platform included data on more than 17 million people in the United Kingdom to identify risk factors for COVID-19 mortality. Authors highlighted the importance of CKD as a risk factor. Using this data platform, Williamson et al. found that patients with GFR <30 ml/min/1.73 m2 had higher risk of mortality from COVID-19 than other patients with hypertension, obesity, or chronic heart or lung disease but without kidney disease.
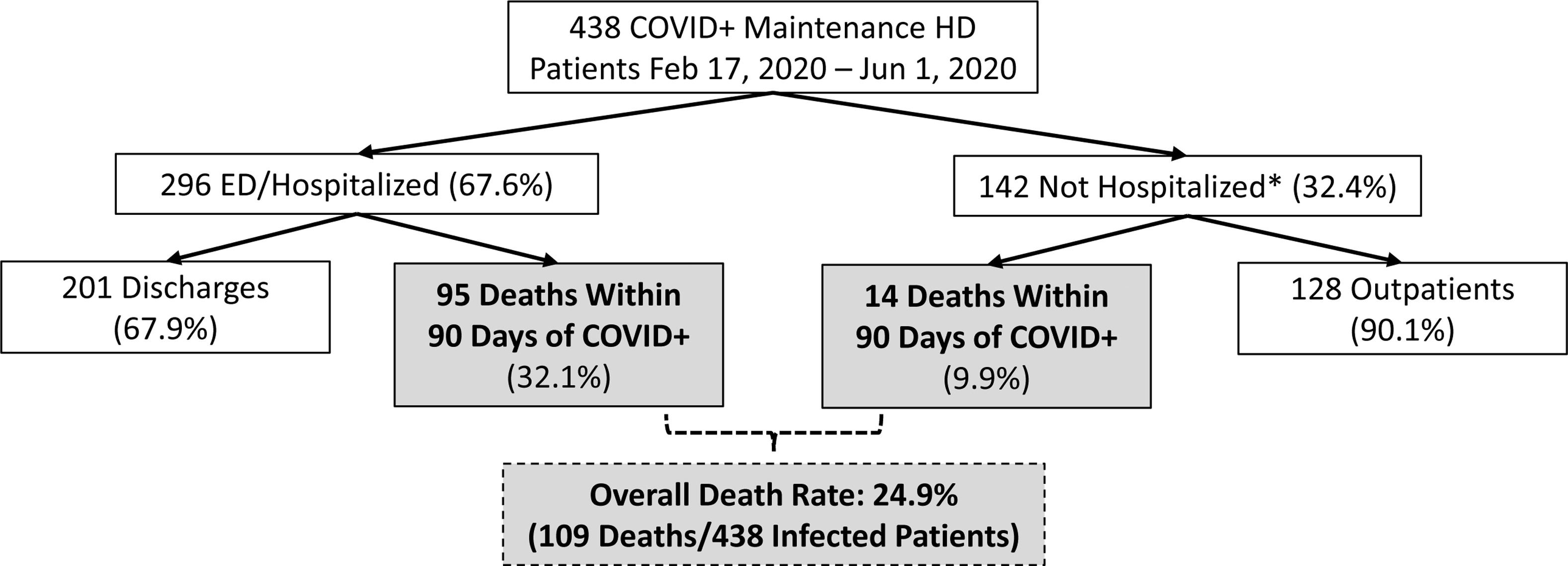
High risk of hospitalization and mortality seen in COVID-19 positive maintenance HD patients from a large national not-for-profit dialysis center in the United States. Older age, heart disease, and markers of frailty were associated with an increased risk of mortality in this study. Figure 1 from Hsu et al, AJKD © National Kidney Foundation.
Importantly, COVID-19 has highlighted existing health disparities and inequities in the US health system. Black, Latino, Native American, and immigrant communities have had disproportionately higher infection and mortality rates compared to the general population. This trend also extends to the kidney failure population. Not unsurprisingly, these racial and ethnic minority patient populations also have lower usage of home dialysis modalities, which have been shown to decrease risk for SARS-CoV-2 infection. Patients who are undocumented immigrants requiring dialysis are particularly vulnerable, as they are excluded from multiple federal programs that pay for standard thrice-weekly outpatient hemodialysis. This has forced many undocumented immigrants to resort to emergency-only dialysis, which has been especially challenging during the COVID-19 pandemic. In the setting of COVID-19 — and wanting to avoid the emergency department — patients tend to delay presentation for dialysis until symptoms, such as shortness of breath, malaise, or nausea become severe. In a population that already endures worse clinical outcomes due to health disparities, this poses an additional challenge and an urgent public health problem. Thus, in addition to implementing measures to prevent SARS-CoV-2 transmission in dialysis centers, increasing access to regular outpatient dialysis and expanding home dialysis options for vulnerable populations should be priorities.
COVID-19 in Transplant

Copyright: Robert Kneschke / Shutterstock
Is it better for a patient to delay transplant and remain on dialysis, or undergo significant immunosuppression and receive a transplant in the setting of a major pandemic? Read this team’s scouting report and decide for yourself!
Currently, there is a paucity of data regarding COVID-19 in kidney transplant patients. The evidence is based largely on case reports and single-center experiences. Here, we highlight the available evidence on topics that are highly relevant to the care of patients with, or awaiting kidney transplants in the setting of the COVID-19 pandemic.
Immunosuppression
Decisions surrounding immunosuppression management in kidney transplant patients who become infected with SARS-CoV-2 should be considered on a case-by-case basis. As with all decisions surrounding immunosuppression, there is a balance between preventing graft rejection and controlling infections. Factors such as baseline graft function, time since transplantation, history of rejection, age, severity of infection, and presence of donor-specific antibodies should be considered. The evidence for immunosuppression management in the setting of COVID-19 is sparse and primarily in the form of case reports. Some experts recommend a 50% decrease in the dose of a kidney transplant patient’s antimetabolites (mycophenolate mofetil, mycophenolic acid, or azathioprine) upon initial COVID-19 diagnosis. This is based on experience with other common viral infections in patients with a kidney transplant, such as BK virus and cytomegalovirus. If clinical status worsens in a patient with COVID-19, antimetabolites can be discontinued entirely.
While many centers have elected to reduce or discontinue antimetabolite therapy, most are maintaining calcineurin inhibitor (CNI) therapy. The reasons for this are multifold. First, CNIs are mainstays of therapy for prevention of graft rejection among transplant patients. Second, there is evidence that tacrolimus may inhibit in vitro replication of coronaviruses. Third, since hyperinflammatory states are seen in severe COVID-19, and since CNIs such as cyclosporine have been successfully used in hyperinflammatory states such as hemophagocytic lymphohistiocytosis (HLH), there has been hypotheses that CNIs may not be harmful and may even be helpful during hyperinflammatory phases of COVID-19. Based on experiences in treating BK virus nephropathy, it has been recommended that tacrolimus doses be adjusted to achieve a trough of 4-6 ng/mL.
When one immunosuppressive agent is decreased or discontinued, steroid doses can be increased to reduce the risk of acute rejection. This is only applicable if patients are not already receiving steroid therapy for their SARS-CoV-2 infection (see above in COVID-19 in kidney failure section). Steroid dosing solely for prevention of graft rejection in a patient with COVID-19 is often left to the discretion of the physicians caring for the patient.
Living and Deceased Donor Transplants
In late March 2020, 72% of US transplant centers reported that they had completed suspended live donor kidney transplantation (LDKT) and 84% had implemented restrictions for deceased donor kidney transplantation (DDKT). An analysis of data from a US transplant registry showed that between March 15 and April 30, 2020, new listings dropped by 18%, DDKT by 24%, and LDKT by 87%. Among states with the highest COVID-19 burden, the number of waitlist deaths was 2-fold greater than expected. The severe decline in transplantation activity was primarily due to concerns over potentially increased susceptibility to COVID-19 and worsened outcomes after SARS-CoV-2 infection among recent transplant recipients(see figure below). However, a single-center study including 56 waitlisted patients and 80 kidney transplant recipients diagnosed with COVID-19 showed that waitlisted patients had significantly higher rates of hospitalization and mortality compared to kidney transplant recipients. Despite DDKT and LDKT returning to pre-COVID-19 levels, the COVID-19 pandemic has had devastating effects on patients awaiting kidney transplantation.

Visual abstract by Michelle Lim on Craig-Schapiro et al.
The risk of donor-transmitted COVID-19 is currently unknown. To date, there have been no proven cases of donor-derived transmission. The American Society of Transplantation (AST) recommends that potential deceased donors be evaluated in terms of residence, travel to areas with high COVID-19 incidence, and recent exposure to COVID-19. Additionally, donors should have had a COVID-19 nucleic acid testing within 3 days of organ procurement. For donors previously infected with COVID-19, the AST recommends consideration for organ acceptance if repeat nucleic acid testing is negative. Guidelines for evaluation of potential living donors are similar. AST recommends but does not require 14 days of self-quarantine prior to donation. At this time, there is no official recommendation to include antibody testing for SARS-CoV-2 in the deceased or living donor screening process.

Barriers to living donor kidney transplant during the COVID-19 pandemic. Figure 1 from Lentine et al, AJKD © National Kidney Foundation.
COVID-19 therapeutic considerations in transplant patients
Overall, the approach to treatment of COVID-19 in kidney transplant patients is similar to that of non-kidney transplant patients. Immunosuppression considerations are discussed above. Currently there are no clinical trials focused on treatment of COVID-19 in kidney transplant patients. A few observational studies have included a small number of transplant patients who received remdesivir, and a number of studies reported the use of anti-interleukin-6 therapy, including one study using the Spanish Society of Nephrology registry that described the experience of tocilizumab use in 80 kidney transplant patients. However, because all patients in this study received tocilizumab, it is challenging to draw meaningful conclusions about the efficacy of this drug in kidney transplant patients.
Outcomes
There are few studies dedicated to examining outcomes after SARS-CoV-2 infection among kidney transplant patients. A recent study using data from the ERA-EDTA Registry found that the mortality risk was 28% higher (HR 1.28 [95% CI, 1.02-1.60]) in transplant recipients compared with matched dialysis patients. Another study using the ERACODA (European Renal Association COVID-19 Database) found that the 28-day probability of death was 21.3% among kidney transplant patients (95% CI, 13.4-30.2%) and 25% in dialysis patients (95% CI, 20.2-30.0%). A prospective cohort study in France enrolled 1216 kidney transplant patients, 66 (5%) of whom were diagnosed with COVID-19. Among patients who were COVID-19 positive in this study, 91% required hospitalization and 24% died. A case series of transplant patients from two centers in the United States found that among 90 solid organ transplant recipients (46 kidney transplant recipients), the mortality rate was 18%. In a systematic review of 24 studies involving 129 kidney transplant recipients with COVID-19, 34% experienced acute kidney injury, 20% of patients required intensive care unit level of care, 25% mechanical ventilation, and 18% died.
Glomerular Injury in COVID-19 vs Tubular Injury in COVID-19
Glomerular Injury in COVID-19

Copyright: gerhardp / Shutterstock
With COVID-19 we have added another item to the list of causes of collapsing glomerulopathy. Patients with high risk APOL1 alleles appear to be at the highest risk of collapsing glomerulopathy, but glomerular injury from COVID-19 may also come from cytokine release, leading to hypercoagulability and thrombotic microangiopathy. Interestingly, we have also seen biopsies in patients with COVID-19 with PLA2R negative membranous nephropathy, IgA vasculitis, lupus nephritis, and anti-GBM disease. Whether or not these are related to the COVID-19 infection is up for debate!
During the course of the pandemic, we have seen glomerular injury in both transplant and native kidneys during COVID-19 infection. Acute kidney injury from glomerular damage may occur in acute glomerulonephritis (GN) as part of a systemic or primary glomerular disease. For example, acute GN has been associated with systemic disease such as bacterial endocarditis, systemic vasculitis, and lupus.

Visual abstract by Verner Venegas on Nasr et al.
In one series of 13 patients with COVID-19, all patients had proteinuria, 85% of which had nephrotic range proteinuria, consistent with glomerular disease. Biopsies in this series consisted of collapsing glomerulopathy in 8 patients, diabetic glomerulosclerosis with acute tubular injury in 3 patients, and one each with PLA2R antibody negative membranous glomerulopathy and diffuse crescentic IgA vasculitis. Another series has demonstrated a similar high incidence of collapsing glomerulopathy with 7 out of 17 patients having this finding on biopsy.

Collapsing FSGS in the setting of COVID-19. Figure 1 from Magoon et al, Kidney Medicine © National Kidney Foundation.
Several mechanisms of kidney damage have been proposed (see figure below) including direct virus-mediated injury, Ang II pathway activation, cytokine storm, and microangiopathy. The main binding site for SARS-CoV-2 is the ACE2 protein, which is found in low levels on podocytes as well as tubules and in the lungs. One hypothesis is that SARS-CoV-2 gains access to the tubules by first binding to the ACE2 protein on the podocytes and subsequently causing kidney damage. The prominence of proteinuria may be explained by viral replication occurring in the podocytes leading to their damage. However, this is still being vigorously debated. In addition, Sars-CoV-2 could lead to ACE2 downregulation, causing increased ANG II accumulation and subsequent AT1 receptor activation. This has been postulated to cause complement activation and hypercoagulability. The ensuing microangiopathy results in fibrin thrombi within glomerular capillaries and glomerular damage.

Various pathophysiological mechanisms associated with COVID-19–related acute kidney injury. Figure 1 from Ng et al, ACKD © National Kidney Foundation.
Severe COVID-19 disease is proposed to be a consequence of a “cytokine storm” or termed immune hyperactivation, and is characterized by an increase in IL-2, IL-6, IL-10, and interferon levels. The dysregulation of the complement system that may occur with a cytokine storm can damage podocytes. For example, IL-6 has been implicated in both kidney autoimmune and inflammatory diseases. Podocytes are unique because they express the IL-6 receptor and, in pro-inflammatory states, this receptor is up-regulated and ultimately leads to a disruption in podocyte differentiation and cell cycle. In addition, IL-1 has been implicated in the exacerbation of toxin-induced AKI.
In proteinuric AKI in COVID-19, biopsy findings have shown a higher than expected incidence of collapsing glomerulopathy. The suspected trigger for collapsing glomerulopathy in COVID-19 is high interferon production. In addition, it is known that patients with APOL-1 high risk genotype are at higher risk for interferon-mediated podocyte injury as seen in lupus, HIV, and interferon-therapy induced glomerulopathy. This is believed to be secondary to a 2-hit hypothesis in which the genetic variant leaves podocytes vulnerable to interferon damage.

Visual abstract by Dhwanil Patel on Wu et al.
Given the immense immune response along with interferon release, it is reasonable to believe that collapsing glomerulopathy may occur in these patients. One small series confirmed the presence of high-risk APOL1 alleles in 6 black patients with collapsing glomerulopathy who underwent renal biopsy for AKI and new onset proteinuria in the setting of COVID-19 infection. Another small series of 6 patients of recent African ancestry with COVID-19, AKI, and proteinuria all had either collapsing glomerulopathy or podocytopathy; 3 patients underwent genetic testing which confirmed the presence of high-risk APOL1 genotypes.
Tubular Injury in COVID-19

Copyright: Vladimir Konstantinov / Shutterstock
Our consult notes frequently read, “AKI (acute kidney injury), likely multifactorial.” In the setting of COVID-19, they should read, “AKI from ATN (acute tubular necrosis). Cause of ATN is likely multifactorial.” ATN from ischemic injury from hypoxemia and/or volume depletion is likely. ATN can also be caused by nephrotoxic injury from direct viral infection of tubules, rhabdomyolysis, and complement mediated injury. No matter the cause, diagnosis of ATN should hold our interest on and off the court!
Although in the early part of the pandemic reports suggested a low incidence of acute kidney injury in the setting of COVID-19, we now know that AKI occurs in a substantial portion of patients with COVID-19. While the incidence of AKI has varied by geographic location and the severity of illness of the patient population being studied, a large meta-analysis found that 17% of mostly hospitalized patients with COVID-19 develop AKI.
Acute tubular necrosis (ATN) has been the predominant lesion described in multiple histopathologic series. In one case series of 26 patients, of those that had a urinalysis available, the vast majority had protein and/or blood in their urine. Every patient in that series was found to have prominent acute tubular injury. This is seen as loss of brush border, dilation of the tubules with cellular debris and vacuolar degeneration. Further, pigmented casts, consistent with rhabdomyolysis, were found in 3 of the cases. In another series of 17 patients with AKI in the setting of COVID-19 who underwent kidney biopsy, acute tubular injury was found in 14 of the patients, and was the most common histologic finding. In another small series of 13 patients with AKI in the setting of COVID-19, all patients had findings of acute tubular injury.

Visual abstract by Omar Taco on Akilesh et al.
The two major causes of ATN are ischemic and nephrotoxic injury, both of which can occur in patients with COVID-19. Patients with COVID-19 often present with acute hypoxia but may also present with nausea, vomiting, and diarrhea with volume depletion. Low oxygen tension and volume depletion can lead to ischemia and therefore ATN.

Acute tubular injury in the setting of COVID-19. Collapsing FSGS in the setting of COVID-19. Figure 1A from Akilesh et al, AJKD © National Kidney Foundation.
The underlying cause of nephrotoxic tubular injury secondary to COVID-19 is still being actively researched, and is likely due to a number of causes including complement activation, direct viral infection, and rhabdomyolysis. SARS-CoV-2 activation of the complement pathway is thought to play a critical role in the pathogenesis of COVID-19, including the development of acute respiratory distress syndrome, AKI, and stroke. In one small series of 6 patients, strong complement C5b-9 (membrane attack complex) deposition was found in the tubules. Direct viral infection also contributes to ATN. The main binding site for SARS-CoV-2 is the ACE2 protein which is expressed on the apical membrane of the proximal tubule. SARS-CoV-2 nucleocapsid protein has been found in the tubular structures of the kidney, and virus-like particles have been seen in tubular epithelial cells. The ACE2 protein is also found in skeletal muscle, and direct viral infection of these cells may lead to muscle damage and rhabdomyolysis leading to ATN as well.
In summary, tubular injury in AKI due to COVID-19 is common and multiple mechanisms of injury are likely. More research is needed to not only determine how SARS-CoV-2 leads to tubular injury but also to develop interventions to prevent long-term kidney disease and kidney failure.
COMMENTARY BY FIONA LOUD:
The year of living sadly
– Executive Team Member for this region: Anna Burgner, AJKDBlog Contributor. Follow her @anna_burgner.
How to Claim CME and MOC
US-based physicians can earn 1.0 CME credit and 1.0 MOC point for reading this region.
- Register/log in to the NKF’s Professional Education Resource Center (PERC). If you select “Physician” in the drop-down menu during registration, the ABIM ID will pop up – make sure to complete this during registration to receive MOC points after course completion.
- Review the activity, disclosure, and accreditation information.
- Click “Continue” and review Course Instructions.
- Complete Post-Test. Please note: By selecting “Yes” to the participation questions for each region, the corresponding Post-Test questions will appear. Click “Save Draft” to save your responses and finish later. When you are ready to submit your answers, click “Preview” to review all responses, then click “Submit.”
- Click “Next” to complete the Evaluation form, then click“Submit.”
- Claim 1.0 CME credit and 1.0 MOC point per region (up to 8.0 total for 8 regions of NephMadness).
- Save/print your certificate.
The CME and MOC activity will expire on June 14th, 2021.



Leave a Reply