#NephMadness 2022: Neonatal Nephrology Region
Submit your picks! | NephMadness 2022 | #NephMadness | #Neonatal
Selection Committee Member: David Askenazi @DDD_Askenazi
David Askenazi is a Professor at the University of Alabama at Birmingham and serves as the Medical Director of the Pediatric and Infant Center for Acute Nephrology. He is the founder and current Board Chair for the Neonatal Kidney Collaborative. Dr. Askenazi’s clinical, educational, and research interests focus on the epidemiology, diagnosis, and renal support therapy of neonates and children at risk for acute kidney injury.
Writer: Michelle Starr @mcstarr1
Michelle Starr is a pediatric nephrologist and health service researcher at Indiana University School of Medicine and Riley Children’s Health. Her research focuses on neonatal kidney disease, including improving identification of kidney injury by providers and families and how kidney disease interacts with other organs such as the lungs. She is a graduate of the 2019 class of the Nephrology Social Media Collective (NSMC) Internship and is a member of the Neonatal Kidney Collaborative executive board.
Competitors for the Neonatal Region
Novel Devices for Neonatal HD vs Novel Therapies in Neonatal AKI
Neonatal AKI and CKD vs Nephron Number and CKD

Copyright: AlexLMX / Shutterstock
Babies have kidneys, too, and you guessed it, acute and chronic kidney disease are bad for babies. Kidney disease in babies is arguably even more concerning given that babies who survive their kidney disease have decades and decades of life in which their kidney problems may become additive. Over the last several years, exciting developments have improved our knowledge of the epidemiology of neonatal kidney disease and have provided insights into interventions that can improve outcomes. Much of this work has been led by the Neonatal Kidney Collaborative, such as the Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study, a 24-center retrospective cohort study which provided additional insights into the epidemiology, risk factors, and outcomes of AKI in neonates.
Major breakthroughs in the last few years in neonatal nephrology include Novel Devices for Neonatal Hemodialysis, methylxanthine to prevent and ameliorate the effects of neonatal AKI, the impact of Neonatal AKI in the life course of kidney disease, and nephron number in the life course of kidney disease. With these new insights, the nephrology community can adamantly say, “It is no longer OK to ignore neonatal kidney disease.”
Novel Devices for Neonatal HD vs Novel Therapies in Neonatal AKI
Novel Devices for Neonatal HD
Acute kidney injury (AKI) is a common complication in infants admitted to the neonatal intensive care unit (NICU). Infants born extremely premature (as young as 22 weeks gestation and <400 g) are surviving to discharge. These infants often have kidney complications, including AKI, during their critical illness. Notably, only 4.1% of infants with AKI in the AWAKEN study were treated with dialytic therapies. Those that require kidney replacement therapy (KRT) have worse outcomes and increased risk of death during their admissions. Although only a small proportion of neonates with AKI receive KRT, it is possible that if therapy were easier to perform, had fewer complications/risks and were more widely available, then more neonates could benefit from KRT.

Copyright: Tomatheart / Shutterstock
Similarly, until recently neonates born with congenital kidney failure requiring kidney support therapy had limited options. Although many pediatric nephrology programs can provide peritoneal dialysis, some situations arise when extracorporeal therapy would be ideal. These include the need for emergent therapy, high dose clearance, careful fluid control, and situations when peritoneal dialysis is not possible (i.e. patients with abdominal infections or recent surgeries).
Initiating dialytic therapy, also referred to as kidney replacement therapy (KRT), can be uniquely challenging in small children. These challenges are multiple and additive. One major challenge is the small size of neonates, which can lead to difficulty with vascular access placement and the lack of appropriately sized central venous catheters for extracorporeal dialytic therapies. To achieve the flows necessary for standard intermittent hemodialysis (iHD) or continuous kidney replacement therapy (CKRT), double lumen central venous catheter of at least 7 French double lumen in size are often needed. This can be a huge challenge in low birth weight or premature infants.
Furthermore, obtaining appropriately placed access, ideally in the internal jugular vein, can be challenging either due to vessel size, clinical stability, or lack of adequately sized vascular access. Outside of the challenges related to the small size of the infant, the machines available to provide therapy to infants provide additional obstacles. The small size and blood volume (estimated to be approximately 70 mL/kg in infants) along with the unique and delicate physiology of this patient population can be easily overwhelmed by changes in electrolyte and fluid shifts which are common in extracorporeal therapies, especially at the time of initiation.
The most commonly encountered challenges in using off-label CKRT machines (currently FDA-approved for larger patients >20kg) are summarized below:
Current Challenges in Using CKRT Machines for Small Patients
| Technical Challenge | Clinical Implication |
| Poor ultrafiltrate accuracy |
|
| Large circuit volumes |
|
| High blood flow (Qb) needed |
|
Recent developments have included refinements in current dialysis machines (such as smaller filters and refinement in peritoneal dialysis techniques) as well as development of novel technologies to address these challenges directly. Some of the most exciting advances and new technologies that have been developed in the last several years are reviewed below:
Cardiorenal Pediatric Dialysis Emergency Machine (CARPEDIEMTM)

The CARPEDIEM machine. Figure 3 from Ronco and Ricci, Kidney News Online, © American Society of Nephrology (reproduced with permission).
The CARPEDIEMTM is the first CKRT machine designed and FDA-approved in the United States for small infants and neonates between the weights of 2.0 and 9.9 kg. The CARPEDIEM machine can perform both hemodialysis and hemofiltration with adjustable and very precise ultrafiltration and blood flow rates. Blood flow can range from 5-50 mL/min (as compared to a minimum of 40 mL/min with the off-label CKRT machines such as Prismaflex).
As this machine is truly designed for smaller patients, it has several benefits which are not available when dialyzing small children with larger CKRT machines. First, two different filter sizes are available with different surface areas (0.15 and 0.25 m2) that can be adjusted based on patient size. Second, gravimetric control allows for finely controlled fluid balance, with an ultrafiltration accuracy within 1 g/h which is essential for appropriately controlling fluid balance in the smallest patients. Finally, an automatic feedback system allows for adjustment in flow rates to keep the difference between prescribed and delivered flow rates less than 20 g/day.
CARPEDIEM recently received FDA device approval for pediatric patients as small as 2.5 kg in the United States, and currently is being used by a handful of pediatric institutions. There have been several recent studies which show that CVVH using CARPEDIEM is well-tolerated and improves metabolic control and fluid overload.
Aquadex
The Aquadex system was initially designed for use in adults with fluid overload from congestive heart failure. Aquadex has been adapted for pediatric patients with AKI and/or volume overload, but adding additional replacement fluids to provide clearance using hemofiltration (CVVH). The Aquadex system has a 33 mL circuit volume and a filter of 0.12 m2 offering a lower extracorporeal option for performing KRT. Additionally, the system has a continuous hematocrit sensor which allows for assessment of intravascular volume status, similar to the hematocrit line often used in intermittent hemodialysis. Monitoring of SvO2 continuously is also available. Ultrafiltration of up to 500 mL/h provides adequate clearance in infants and small pediatric patients.

Aquadex schematic diagram. Figure 2 from Raina et al, ACKD, © National Kidney Foundation.
There have been three studies published which evaluate use of Aquadex efficacy in pediatric patients. The largest of these, published by Menon and colleagues in 2019 which included 117 patients at 3 pediatric institutions, reported higher survival rates than traditional CKRT modalities. In their cohort of patients weighing <10 kg, 60% survived until end of treatment or transition to another modality (such as a switch the peritoneal dialysis) and 32% survived to hospital discharge. In those >20 kg, survival to end of treatment was 97% and survival to hospital discharge was 68%. Complications were noted in 15% of treatment sessions, and were primarily due to vascular access challenges. Only 3% had hemodynamic instability with circuit initiation/changes. In 2021, Aquadex was approved by the FDA for patients > 20 kg with fluid overload, and is currently being used off-label for CVVH in small children in about a dozen pediatric academic centers.

Visual abstract by @divyaa24 on Menon et al.
Newcastle Infant Dialysis and Ultrafiltration System (NIDUS)
Another innovative approach to dialysis in the smallest patients is proposed by the NIDUS system, which is a syringe-driven machine which repeatedly withdraws small aliquots (5-12.5 mL of blood) from a single lumen central venous line (CVL) at a rate of 20 mL/min. Because the system processes 5 mL of blood every minute and has a total circuit volume of only 4-10 mL depending on the stroke volume, it does not require a blood prime even in the smallest of patients. Additional benefits of the NIDUS platform include greater control of fluid balance as ultrafiltration control is controlled using volumetric and direct control. Furthermore, the system can be used in a continuous manner to allow for CKRT if needed.
Despite these novel advances, NIDUS remains in need of further clinical trials to assess its efficiency and applicability to infants and small pediatric patients. A crossover trial to evaluate the utility found adequate clearance of small- and medium-sized molecules in infants <8 kg. An ongoing Infant Kidney Dialysis and Filtration (I-KID) study, a multicenter, randomized clinical investigation using a cluster stepped-wedge design with one-way crossover study with each unit acting as their own control, is underway and should further evaluate the role of NIDUS in this patient population.
HF-20 Membrane
Finally, the HF20 filter has been recently approved by the FDA. This filter has a relatively low circuit volume (65mL) and made of polyacrylethersulfone, both characteristics which decrease the number of patients that require a blood prime as well decreasing the risk of bradykinin release syndrome (which was frequently seen with the smallest membranes used for CKRT due to the polyacrylethersulfone composition). This filter can be used in the most commonly used pediatric CKRT machines (Prismaflex) and now the Prismax machines. In the several studies that have reported on the use of HF20 filters, it appears that the filter offers adequate and reliable therapy in critically ill pediatric patients with fluid removal as targeted.
| CARPEDIEM | Aquadex | NIDUS | Prismaflex with HF-20 |
|
|
|
|
In conclusion, the neonatal population deserves size-specific machines, vascular access and filters to provide effective and safe KRT. Patients with AKI and congenital kidney failure are benefiting from these new technologies. The last several years have heralded dramatic advances in neonatal KRT – including three machines which have the potential to revolutionize care to the smallest patients. Additionally, the FDA has recently been supportive of additional specific pediatric and neonatal devices in order to provide KRT to smaller patients, increasing their access and use at pediatric institutions. These devices are paving the way into the future of neonatal nephrology, allowing nephrologists, neonatologists and intensivists to provide better therapy for the smallest patients with kidney disease.
COMMENTARY BY SHINA MENON:
Novel Devices in Neonatal Nephrology –
The Times They Are a-Changing
Novel Therapies in Neonatal AKI
Is it possible to either treat AKI in neonates or prevent it before it even occurs? Are methylxanthines the answer? While the search for a treatment or intervention for neonatal AKI has remained elusive overall, several agents have been evaluated in neonatal cohorts with exciting results. Some of these have shown early promise, especially in conditions like perinatal asphyxia and heart surgery where the timing of the insult is known to some degree. Here we describe two methylxanthines – theophylline and caffeine – with similar mechanistic properties which have shown promise and are exciting potential therapies for management of neonates with AKI.

Copyright: stockcreations / Shutterstock
Theophylline
Theophylline and its related salt aminophylline have shown some success in increasing urine output and decreasing the severity of AKI in small studies of mostly term and post-term infants with perinatal asphyxia. Perinatal asphyxia (hypoxia in a newborn due to challenges relating to delivery or maternal complications) is a high-risk event that often leads to multi-organ dysfunction and AKI in newborns. One treatment commonly used is therapeutic hypothermia, either whole body or selective head cooling. Animal models and studies in humans report an increase in kidney adenosine during episodes of kidney hypoxemia and ischemia. This then leads to kidney vasoconstriction and subsequent decreased glomerular filtration rate and urine output. Theophylline, an adenosine receptor antagonist, thus has a potential mechanism for prevention of AKI in asphyxiated neonates.
Several studies, all in babies who did not receive therapeutic hypothermia, have shown that a single dose of theophylline given prophylactically within six hours of birth is associated with more urine output and lower creatinine rise in the first five days of life. Aminophylline is a salt of theophylline with the same active molecule, and has also been evaluated in a small retrospective cohort of neonates with perinatal asphyxia. In that study, infants treated with aminophylline had higher urine output and greater decline in serum creatinine within 12 hours of aminophylline than those who were not treated. Of note, the babies in the study did receive therapeutic hypothermia as part of routine care. In addition, aminophylline, which is used in this center as part of clinical care, was given only to babies who were showing signs of AKI including decreased urine output and rising serum creatinine rather than uniformly as prophylaxis. Interestingly, the babies who showed a response to aminophylline survived whereas the non-survivors did not have increased urine output following treatment.
The first meta-analysis on this topic included four randomized controlled trials and 197 infants (term and post-term) with severe hypoxia who were treated with therapeutic hypothermia. The overall pooled RR for developing severe acute kidney injury in the theophylline-treated group was 0.38 (95% CI, 0.25-0.57; P < 0.001). The effect of theophylline on urine output and serum creatinine was greatest on days three and five. Three of the 4 trials also showed a significant reduction in levels of urine β2 microglobulin, a biomarker of tubular function and injury. A subsequent meta-analysis further confirmed these findings. No significant difference in adverse events were reported in the theophylline group compared with controls.
Based on the evidence available from existing meta-analyses, a one-time dose of theophylline within the six hours after delivery for infants with perinatal asphyxia is endorsed in the KDIGO guidelines. It is important to note that theophylline does have a significant side effect profile, which includes seizures, arrhythmias, and gastrointestinal complications. However, none of the studies that have been performed noted any difference in either adverse events between infants receiving theophylline and control groups. Certainly, these findings should be further evaluated in larger, randomized control trials including in infants treated with whole body cooling, which has become the standard of care in neonates with perinatal asphyxia (as opposed to head-only cooling, which was more common at the time of these studies). Additionally, further work should evaluate the long-term kidney outcomes in these infants.
Caffeine
Caffeine, a methylxanthine-like theophylline, is an adenosine receptor antagonist that has been evaluated regarding its possible protective effects against AKI in pre-term cohorts. Caffeine is commonly given to premature neonates to improve respiratory drive and to decrease the risk of apnea of prematurity. Two studies have shown that AKI occurred less frequently in prematurely-born infants who received caffeine within the first week of age as compared to those who did not receive caffeine. In the first study, Carmody et al evaluated the effect of caffeine exposure on AKI rates in a retrospective cohort of 140 very low birth weight neonates. AKI occurred less frequently in those who received caffeine compared with those who did not (18% caffeine/AKI vs. 44% no caffeine/AKI, P = 0.002). In a larger retrospective cohort (n = 675) using AWAKEN data, Harer et al demonstrated similar findings. In AWAKEN, AKI occurred less frequently in neonates who received caffeine in the first week of life than in those who were not treated with caffeine (11.2% vs. 31.6%, P<0.001).
Importantly, the infants who received caffeine in this study had more risk factors for AKI including lower average gestational age, lower birth weight, and high severity of illness scores. Despite this, they were still less likely to develop severe AKI. Based on this study, the number needed to treat (NNT) with caffeine to prevent one episode of AKI was 4.3. However, there is not yet any long-term data on the role of caffeine on CKD or long-term kidney health. Therefore, studies which evaluate the long-term kidney outcomes in these infants are also needed.
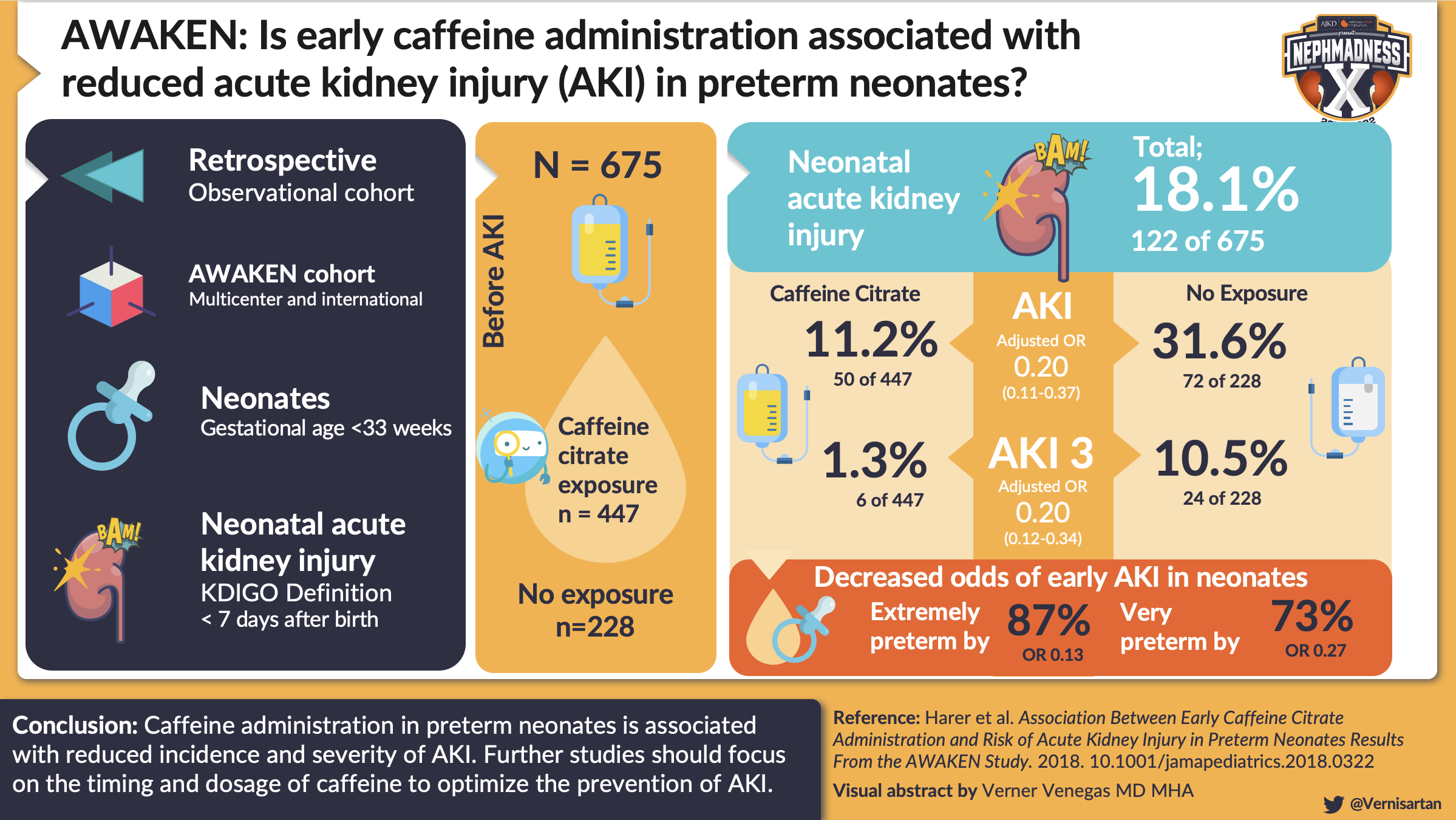
Visual abstract by @Vernisartan on Harer et al.
These studies and recent advancements in the field raise the question – could novel approaches to therapy with already available medications such as caffeine and theophylline prevent or treat AKI in neonates in certain clinical situations? While more studies are certainly needed, the advances in the treatment and prevention of neonatal AKI are driving new research and exciting researchers and those that care for neonates alike.
Check out this podcast episode of The Cribsiders featuring Michelle Starr @mcstarr1:
#46 Neonatal AKI
Neonatal AKI and CKD vs Nephron Number and CKD
Neonatal AKI and CKD
One of the major outstanding questions in neonatal nephrology is how neonatal AKI impacts children during their life course, including its impact on increased risk of CKD and hypertension.

Copyright: Dream79 / Shutterstock
Neonatal AKI (AKI) is common in critically ill neonates as well as premature neonates, and an episode of AKI appears to decrease nephron number and potentiate progression to CKD. This increased AKI risk in critically ill neonates is due to many factors, including impaired vasoregulation of the glomerulus, low GFR during the first several weeks following delivery, tubular immaturity, and exposure to nephrotoxins. While the incidence of neonatal AKI has long been challenging to determine given variable definitions, a recent multicenter retrospective cohort study reported an astonishingly high incidence of AKI of 48% in those born before 29 weeks of gestation (based on neonatal modified KDIGO criteria, when AKI was defined using a standardized definition of serum creatinine rise of ≥0.3 mg/dL (26.5 mcmol/L) or ≥50% from previous lowest value, and/or if urine output was <1 mL/kg/h during the first week following birth).
It had long been thought that those who survive an episode of AKI (neonatal through adulthood) recovered kidney function without long-term sequelae; however, over the last decade, data from animal models, critically ill children, as well as adults with AKI suggest that survivors are indeed at risk for development of CKD. Additionally, as premature neonates may be undergoing postnatal glomerulogenesis during the time of their AKI, these episodes are thought to act as a “second hit” and alter kidney development and/or nephron mass potential. In human autopsy studies, postnatal development was affected by occurrence of AKI as well as exposure to nephrotoxic medications, such as gentamicin, vancomycin and non-steroidal anti-inflammatory drugs. Additionally, in neonates with AKI there is evidence of accelerated kidney maturation, abnormally formed and hypertrophied glomeruli compared to gestational age matched controls, and a decreased number of immature glomeruli.
Several studies in pediatric patients have shown that AKI is associated with the development of CKD. One long-term follow-up study of 126 critically ill children with AKI without pre-existing CKD found that during the 3-year follow-up period, 40% of children developed CKD. Of note, this number was much higher in the small subset of neonates in their cohort (70%). Another recent study found that children with nephrotoxin-associated AKI had a high risk of CKD, as 70 of the 100 patients had residual kidney damage 6 months after discharge.

Visual abstract by @divyaa24 on Menon et al.
Studies about the long-term implications of AKI and associations with CKD in neonates have so far been limited to small single center retrospective reports. Compared to term neonates, premature and low-birth weight infants with AKI have twice the rates of albuminuria, CKD, and end-stage kidney disease (4.5-18 years after birth). A meta-analysis of 8 longitudinal studies evaluating kidney function of children diagnosed with AKI found that among the 293 children followed, 53 (18%) had evidence of CKD, and a long-term follow-up study assessed kidney function in 34 premature infants with and without AKI at age 3–7 years found that infants with nAKI had a higher likelihood of kidney dysfunction (65% vs. 15%) based on GFR, elevated urinary protein excretion, or systemic hypertension.
Summary of studies on the long term implications of nAKI
| Study | Population | AKI Definition |
Findings |
| Abitbol et al | Low birth weight neonates <1 kg
Follow-up at 7.5 years All with AKI |
Peak Cr >
2.0 mg/dL for 48 h or UOP <0.5 for 24 h |
55% with low eGFR (Cr) and 25% with ESKD (4 needing transplant) |
| Bruel et al | Premature <33 weeks
Follow-up at 6.5 years |
Cr >
1.6 for 24-27 wk 1.1 for 28-29 wk 1.0 for 30-32 wk |
Kidney volumes lower in those with AKI, no difference in albuminuria, eGFR |
| Maqsood et al | Low birth weight neonates <1 kg
Follow-up at 3 years |
Modified KDIGO | No difference in CKD |
| Harer et al | Low birth weight infants <1.5 kg
Follow-up at 5 years |
Modified KDIGO | AKI Group with more kidney dysfunction (65% versus 16%) |
Although much more remains to be learned about the long term implications of AKI and other post-natal insults in neonates, the growing evidence suggests that AKI accelerates post-natal nephrogenesis and may lead to a greater decline in kidney function. However, the lack of appropriately powered studies, consensus definitions for AKI and CKD, and consistent follow-up period are significant barriers to clearly defining the associations between AKI and subsequent CKD. Thus, large multi-center long-term follow-up studies of neonates at greatest risk for AKI are needed in order to clarify the underlying risk of CKD development during childhood and adulthood in these high-risk patients.
COMMENTARY BY MICHELLE RHEAULT:
New and Old Therapies for the Tiniest Kidneys
Nephron Number and CKD
Approximately 15 million infants worldwide are born prematurely (<37 weeks of completed gestation) each year and complications from premature birth account for approximately 1 million deaths each year. Dramatic improvements in neonatal intensive care, advancements in our understanding of neonatal physiology, and implementation of therapies (such as prenatal glucocorticoids and surfactant replacement) have led to improved survival of premature infants. These include a growing number of infants born extremely premature or at very low birth weight. The most recent data from the Vermont Oxford Network suggest that approximately 90% of infants with a birth weight between 500 g and 1,500 g survive to discharge.

Copyright: Lukasz Szwaj / Shutterstock
There are two hypotheses that form the framework for reduced nephron number and its impact on kidney health throughout the life course. The first, that of prenatal origins of adult disease, is credited to David Barker who made the observation that many “adult” diseases appear to have their origins in fetal life. This conclusion, drawn initially from epidemiologic associations between low birth weight and adult hypertension, is commonly called the Developmental Origins of Health and Disease (DOHaD) hypothesis. Barry Brenner extended this principle to kidney development, suggesting that fetal stressors result in reduced nephron number at birth, predisposing individuals to CKD.
Human nephron number is highly variable, ranging from 210,000 to 2.7 million, with this variability hypothesized to contribute to an individuals’ susceptibility to kidney disease. The reasons for this variability are unknown. Kidney development continues in neonates until 34-36 weeks of gestation with more than 60% of nephrons formed in the last trimester of pregnancy. Nephrons do not regenerate; therefore, the nephrons present in neonates at time of birth must last a lifetime. Even in healthy adults who are born at full-term, the number of nephron units decrease slowly over time, leading to an age-dependent decline in glomerular filtration rate (GFR).
Brenner’s theory, which is also referred to as “nephron under-dosing”, suggests that over time individual nephrons increase their available surface area to compensate for decreased nephron number, an adaptive response that becomes maladaptive. Glomerular surface area increases leading to systemic hypertension and sodium retention, disrupting kidney auto-regulatory mechanisms and worsening proteinuria and hypertension. This in turn leads to nephron sclerosis, resulting in additional decline in nephron number and more rapid nephron dropout.
To date, there are no prospective, population-based studies that confirm the association between prematurity and CKD later in life. The original studies by Barker as part of the DOHaD hypothesis did not attempt to distinguish between premature birth and intrauterine growth restriction (IUGR) as the cause of low birth weight (LBW). Examining the impact of prematurity alone is challenging as many infants born prematurely also have IUGR, as well as multiple medical problems and medication exposures during their perinatal and postnatal courses, including AKI and nephrotoxins. Despite these confounders, studies have repeatedly demonstrated strong associations between fewer glomeruli and a higher risk of proteinuria, hypertension, salt sensitivity of blood pressure, and progressive CKD.
The best-studied marker for adverse intrauterine environment is LBW. A meta-analysis including more than 2 million individuals from 31 studies found that LBW was associated with an approximately 80% increased odds of albuminuria, 80% increased odds of a sustained low eGFR, and an approximately 60% increased odds of kidney failure in later life compared to their normal birth weight counterparts. The largest study to analyze this association was a Norwegian registry-based study conducted from 1967 to 2004 which found a relative risk of 1.7 for the development of ESKD for all infants with birth weights <10th percentile. A large national registry-based study including over 20,000 persons born from 1924 to 1944 and followed until death found that both prematurity and LBW were associated with increased risk of CKD (based on ICD-9 codes), with infants born less than 34 weeks having a 2.6-fold increased risk of developing CKD.
Studies of long-term kidney function following premature birth have been completed in children and adolescents. Despite this relatively short period of follow-up, several studies have described associations between premature birth and increased urinary albumin excretion and reduced eGFR. Among a cohort of adolescents born prematurely, infants with LBW were 1.4 times more likely to have microalbuminuria and a decreased eGFR, and those who also had IUGR had a 2.4 fold increase in albuminuria. Additionally, infants born prematurely appear to have smaller kidneys on renal ultrasound which may reflect decreased nephron number compared to their age matched controls. A study published in BMJ found that preterm and early term birth were strong risk factors for the development of CKD during adulthood.

Visual abstract by @iheartkidneys on Crump et al.
While changes in kidney function in adolescence or young adulthood are often subtle, these abnormalities may progress to overt kidney dysfunction during adulthood or leave children susceptible to secondary kidney injuries during their life course. Both pediatric and adult nephrologists should be aware of prematurity as an important risk factor for CKD, hypertension, and kidney problems throughout the life course, and all nephrologists should include a birth history in their initial assessment!
– Executive Team Members for this region: Anna Burgner @anna_burgner and Matthew Sparks @Nephro_Sparks
How to Claim CME and MOC
US-based physicians can earn 1.0 CME credit and 1.0 MOC per region through NKF PERC (detailed instructions here). The CME and MOC activity will expire on June 1, 2022.
Click to read more NephMadness 2022 Regions


Leave a Reply