#NephMadness 2025: Hemodialysis Region
Submit your picks! | @NephMadness | @nephmadness.bsky.social | NephMadness 2025
Selection Committee Member: Mariana Murea @MarianaMurea
Mariana Murea is an Associate Professor, nephrologist, and clinician-scientist at Wake Forest University School of Medicine. Her research program focuses advancing patient-centered dialysis care, addressing fundamental questions such as the independent impact of arteriovenous access type on clinical and patient-reported outcomes and determining the optimal strategies for initiating hemodialysis.
Writer: Manasi Bapat @manasib33
Manasi Bapat is a nephrologist at Kaiser Permanente, Walnut Creek, California. Her clinical interests include hypertension and glomerular diseases. She is actively involved in Free Open Access Medical Education (FOAMed) through her collaborations with GLomCOn and NephJC. She is also a part of Nephtrials – a joint initiative between NephJC and ISN’s Advancing Clinical Trials to increase nephrology clinical trial awareness and participation worldwide.
Competitors for the Hemodialysis Region
Team 1: Hemodiafiltration
versus
Team 2: Incremental Dialysis

Image generated by Evan Zeitler using DALLE-E 3, accessed via ChatGPT at http://chat.openai.com, February 2025. After using the tool to generate the image, Zeitler and the NephMadness Executive Team reviewed and take full responsibility for the final graphic image.
We live in the era of “precision medicine” – one of the most exciting innovations in medicine today. Its goal is to depart from the one-size-fits all approach and individualize treatments to each patient, taking into account differences in people’s biology, genetics, and socioeconomic determinants. In accordance with this goal, a “Framework on integrated, people-centered health services” was put forth by the World Health Organization (WHO) in 2015 making a push to ensure that health services are tailored to people’s needs and are provided in partnership with the patients, rather than a focus purely on the disease.
Considering the goals of patient- or people-centered medicine, we seem to be pretty far away from it, especially when it comes to the management of patients with advanced kidney disease treated with chronic hemodialysis (HD). More often than not, patients initiating HD are prescribed a standard three times per week 3.5 hours of high-flux HD treatment targeted to Kt/V to measure adequacy, with an intense focus on parathyroid hormone (PTH) and phosphorus control and relatively less focus on disease heterogeneity, patients’ ability to constantly submit to rigid schedules, and their quality of life.
The first dialysis treatment took place in 1943 by Dr. Kolff; while we have come a long way from the rotating drum cellophane dialyzers, patients on chronic dialysis continue to experience dismal outcomes, with median survival rates as low as 50% in 5 years going hand in hand with high rates of hospitalizations and poor quality of life. We have tried to tweak the treatment of HD with various interventions–e.g., higher dose HD, more frequent HD, cool dialysate HD–but so far none of these interventions has made headway towards improving outcomes.
“You have to be able to accept failure to get better” – LeBron James
Let’s consider two approaches to dialysis therapy aimed at improving outcomes for patients undergoing chronic HD. First, hemodiafiltration (HDF) is gaining recognition as a superior treatment beyond traditional high-flux HD. Additionally, incremental-start HD is emerging as a patient-centered strategy for initiating chronic dialysis, prioritizing individualized care.
In this NephMadness HD Region, we have a compelling matchup between HDF and incremental HD. We will examine which patient populations are best suited for these dialysis strategies, review the supporting evidence, and explore the barriers that may hinder their broader adoption.
Team 1: Hemodiafiltration

Copyright: OlegKovalevichh/ Shutterstock
Efforts to address the limitations of HD: i.e., inadequate clearance of middle and large molecular-weight molecules led to the conception of HDF. A deeper dive into the history of HDF leads us to the pioneering investigations Henderson and Leber, who coined the term HDF in 1977-78.
HDF combines diffusion, which provides effective removal of small molecules, and convection, which provides improved clearance of larger molecules achieved using hemofiltration. In the early days of HDF, commercially-prepared replacement fluid in bags was used that allowed a “low volume” reinfusion rate of 9L per session. The replacement fluid refers to a sterile, specially produced solution that is infused back into a patient’s bloodstream during treatment to replace the large volume of plasma water removed through ultrafiltration. This is essential to maintain fluid balance while removing waste products from the blood and is a key component of the HDF process. “High volumes” of non-pyrogenic pure and safe water “on-line”(OL) can now be produced in outpatient dialysis units in real time and continuously, thanks to technological developments. This allows for the production of fresh ultrapure dialysate, which can then be reinfused as replacement fluid, enabling OL-HDF with high fluid turnovers of up to 30-40L per session.
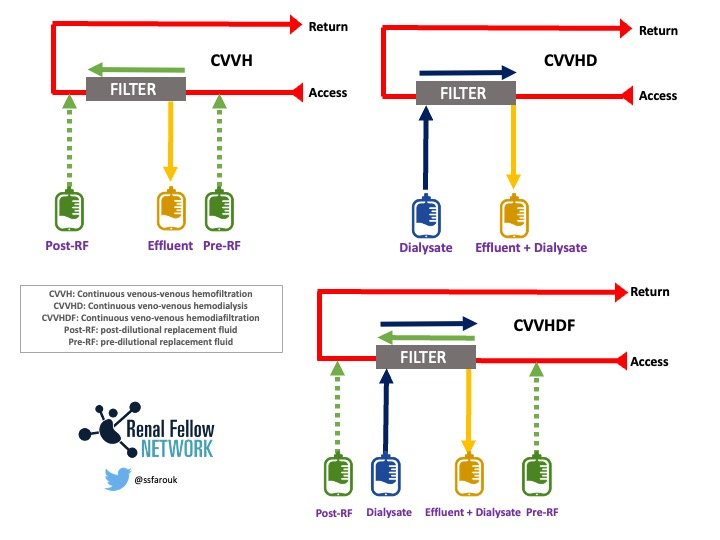
Comparison of Circuit Setup Hemofiltration, Hemodialysis, and Hemodiafiltration. Figure courtesy of Samira Farouk, Renal Fellow Network.
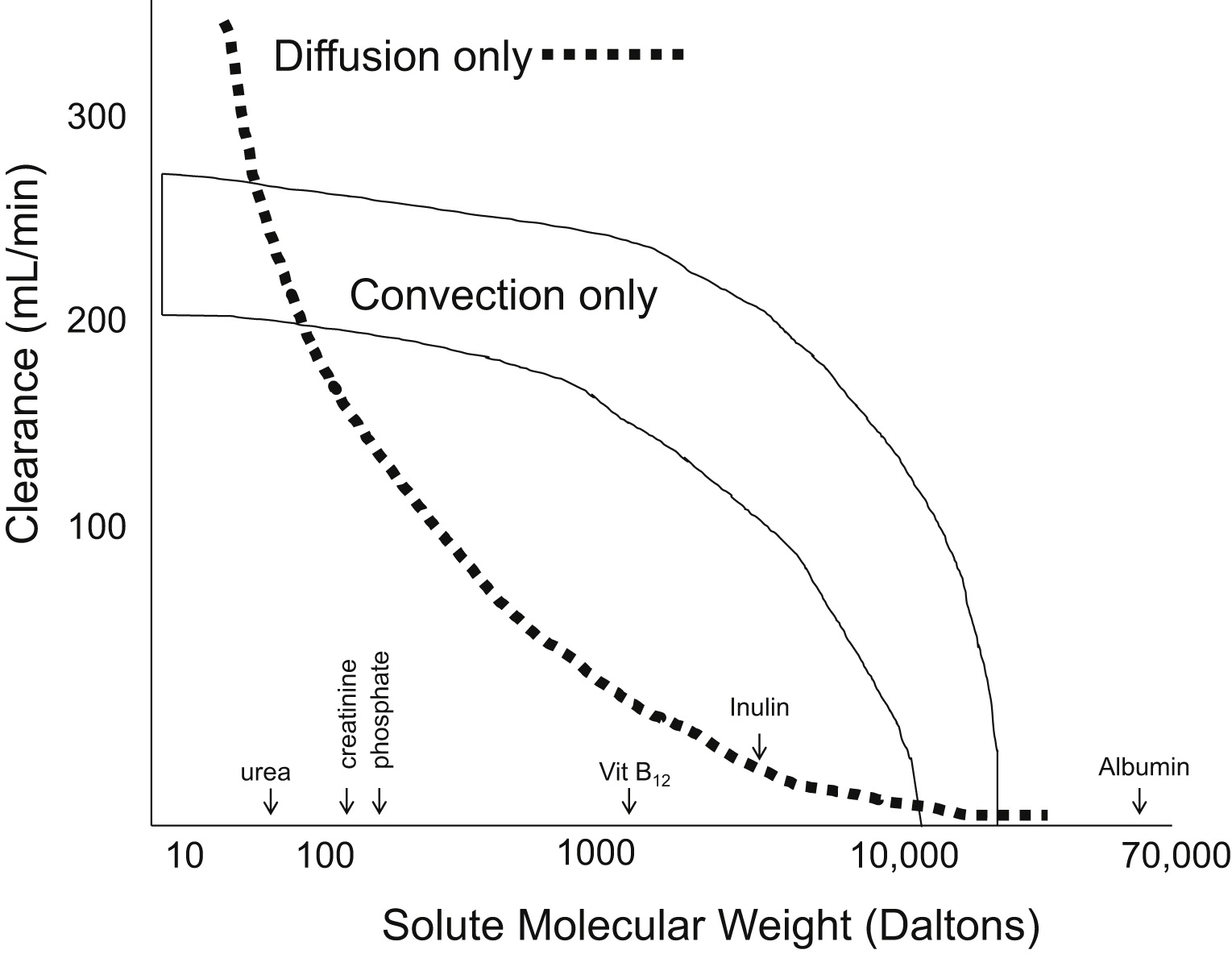
An approximation of the difference in clearance between pure diffusive processes and pure convective process by the molecular weight of the solute. Fig 1 from Golper et al, © National Kidney Foundation.
Middle to large uremic toxins are thought to contribute to increased cardiovascular (CV) mortality and impaired immune function in patients undergoing chronic dialysis. Observational studies have indicated that The use of convective therapies has been indicated to reduce the incidence of manifestations of beta-2 microglobulin amyloidosis, including carpal tunnel syndrome. Removal of FGF23 was markedly higher during HDF. Since FGF23 in patients on chronic dialysis has been associated with left ventricular hypertrophy (LVH) and CV events, removal has been proposed as a potential mechanism of lower CV mortality with HDF. Studies on HDF have shown improved clearance of advanced glycation endproducts (AGEs) attributed with endothelial dysfunction and CV mortality. However, it is still uncertain if the improved clearance of these molecules per se influences clinical outcomes in HDF. Furthermore, observational studies were fraught with potential confounding, as some determinants of survival are also factors in received volume, and because many of the dose results are post hoc or per protocol (and not intention to treat) analyses.
Five large randomized controlled trials (RCTs) have attempted to determine the relative merits of HDF and HD in regards to relevant clinical endpoints of all-cause mortality, CV mortality, and infection-related morbidity or mortality with mixed results.
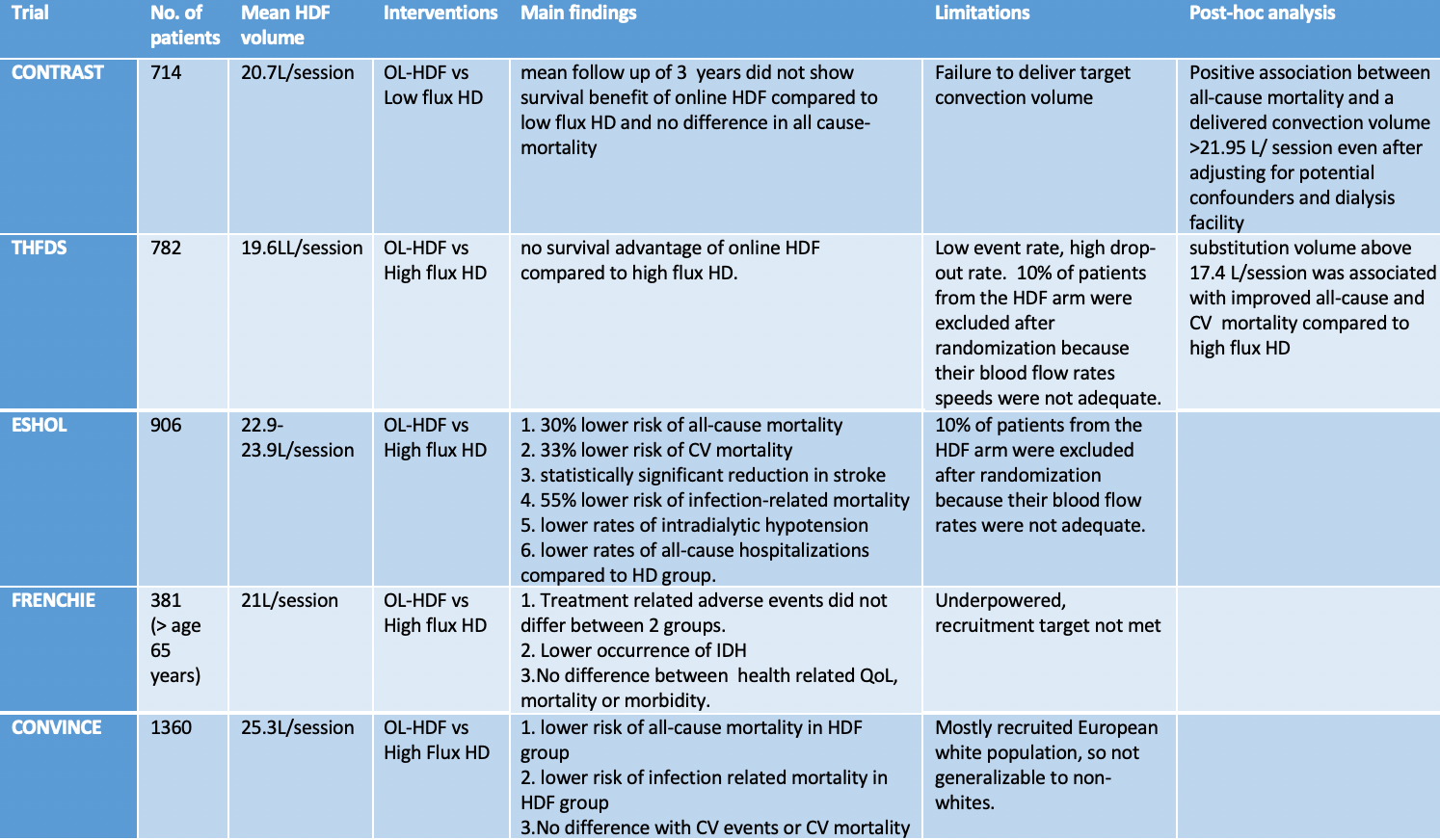
Results of the CONTRAST, ESHOL, THFDS, FRENCHIE, and CONVINCE trials.
Figure adapted and modified from NephJC: H4RT
Peters et al conducted an individual participant data (IPD) meta-analysis of the first four RCTs in the table, and showed that OL-HDF was associated with a 14% reduction in all-cause mortality and a 23% reduction in CV mortality compared to HD. The survival benefit was most pronounced in patients receiving higher delivered convection volumes (>23 L per 1.73 m² per session). Long-term follow-up of these four large RCTs indicated that Left Ventricular mass and ejection fraction (EF) tended to worsen in the HD group, while remaining stable in OL-HDF patients, suggesting a long-term cardioprotective effect of OL-HDF.
Reviewing all the data from observational studies to RCTs to meta-analyses in HDF feels like a game of tug of war!
Previous RCTs comparing the effectiveness of HDF vs. HD have had substantial design heterogeneity, making it challenging to draw definitive conclusions. A well-designed trial was needed to rigorously assess whether HDF offers superior outcomes, particularly regarding CV mortality, and to determine the optimal convective dose across different clinical settings. The CONVINCE study, published in 2023, marks a significant milestone in evaluating OL-HDF. It reported a lower all-cause mortality in the HDF group (17.3% vs. 21.9% in HD), yet found no significant difference in CV mortality between the two groups. So, has the CONVINCE trial provided enough evidence to integrate HDF into clinical practice guidelines?
An updated meta-analysis that included data from the CONVINCE trial further confirmed a lower all-cause mortality in the OL-HDF group (23.3%) compared to the HD group (27%). Additionally, OL-HDF demonstrated better intradialytic hemodynamic stability compared to high-flux HD.
Regulatory barriers have limited the availability of HDF in the USA, resulting in most RCT data on HDF coming from European studies. In the USA, HDF delivery systems must generate on-line, ultrapure, non-pyrogenic substitution fluid, which the FDA classifies as a medical device requiring clearance before patient use. To address these challenges, the American Society of Nephrology (ASN) and the FDA launched the The Kidney Health initiative (KHI). This partnership aims to facilitate the development and approval of innovative kidney therapies in the USA, including exploring pathways for introducing treatments like HDF that are already established in Europe and Canada.
An unresolved issue surrounding HDF is its cost effectiveness. The quality-adjusted life year (QALY) is the academic standard for measuring how well different kinds of medical treatments lengthen and/or improve patients’ lives. It is a metric that serves as a fundamental component of cost-effectiveness analyses. Cost utility analyses (CUA) measure health effects in terms of both quantity (life years) and quality of life. These are combined into a single measure of health, i.e, the QALYs.
A prior probabilistic sensitivity analysis found that HDF is cost effective; however, a 2013 CUA suggested that, while the additional costs of HDF appear minimal compared to HD, the limited QALY gain does not justify its use as a cost-effective treatment at present.
A much-anticipated cost utility analysis from the CONVINCE trial, presented as a late-breaking poster at Kidney Week 2024, compared OL-HDF to HD. The results indicated that HDF provides an additional year of health at higher costs. The probability of HDF being cost effective is largely influenced by dialysis costs and life years gained. At a €60,000/QALY threshold, the likelihood of HDF being cost effective exceeds 75%, though costs may vary by country. This means that in a cost-effectiveness analysis, HDF would be considered cost effective if it costs less than €60,000 to produce one additional QALY compared to the standard treatment.
The Haemodiafiltration versus High-flux Haemodialysis Registry Trial (H4RT) is another large, ongoing RCT whose primary objective is to assess the effectiveness of high-volume HDF compared to high-flux HD on non-cancer mortality; a key secondary outcome is to compare the cost effectiveness of high-volume HDF versus high-flux HD. This trial is slated to end in September 2025.
Several important questions remain unanswered regarding OL-HDF. While conceptually high-volume HDF offers advantages over low-volume HDF in terms of increased diffusive and convective clearance, no studies have yet directly compared the two approaches. Most existing studies on HDF have focused on prevalent dialysis patients, but what about those starting chronic dialysis who have residual kidney function (RKF)? How should we determine the optimal convective volume for these patients?
Some may argue that the full potential of OL-HDF to improve outcomes in chronic dialysis patients, particularly in terms of all-cause and CV mortality, remains unclear. Notwithstanding this argument, there is growing evidence that we are getting closer to understanding its true impact. Efforts to make this treatment more widely available are progressing, and we may soon see its broader adoption in clinical practice.
In this podcast episode of GN in Ten, hosts Joel Topf, Jordy Cohen, and Nayan Arora are joined by clinician/scientist Mariana Murea and nephrologist Katie Kwon.
FF 78 NephMadness, the dialysis region
Team 2: Incremental Dialysis

Copyright: gemphoto/ Shutterstock
Incremental-start HD, or commonly called incremental HD, involves adjusting HD frequency or treatment duration based on a patient’s biology and clinical manifestations. This approach embodies a “patient-centered” model, as it enables dynamic and individualized adjustments to the HD prescription, considering factors such as RKF, blood pressure, volume status, and adequacy measures, among others. Consequently, the initial HD prescription can vary significantly from the standard “thrice-a-week HD,” with alternatives like twice-a-week HD or three sessions per week with shorter treatment durations. One might think that initiating patients on HD treatment with less time or frequency might be a reasonably simple endeavor, but hold thy breath because it’s not. The plot thickens!
In the late 1960s, with the advent of chronic HD, treatments were offered on varying schedules based on patients’ clinical manifestations. However, chronic HD soon evolved into a one-size-fits-all model, with a minimum of thrice-weekly treatments and a set minimum dose for each session. As a result, incremental HD gradually faded from clinical practice. In a nationally representative sample of patients starting chronic HD, 434 were initiated on incremental HD, while 50,162 were started on the conventional thrice-weekly HD regimen. Of the 1,737 dialysis facilities across the United States, 74% (1,293) did not have any patients treated with incremental HD between January 2007 and December 2011. Meanwhile, 17% (288) of facilities had between 0% and 3% of incident HD patients receiving incremental HD, and 9% (156) of facilities had more than 3% of their incident population on this regimen. Clearly, maintaining or re-embracing the “less is more” paradigm of incremental HD has been challenging and slow. But why?
The “optimal” dialysis dose in conventional HD was derived from the NCDS study and the HEMO study that involved prevalent HD patients with low or negligible RKF. Based on these trials, incident and prevalent patients on HD are, by and large, treated with three times a week HD to target a minimum dialysis single pool Kt/V urea (spKt/V) of 1.2 without regard for RKF. However, many patients who transition from pre-dialysis CKD to dialysis initiation have appreciable levels of RKF. Nevertheless, to date, no large RCTs have been completed that specifically study patients who start chronic HD with RKF to directly compare standard thrice-weekly HD with incremental HD.
In a thought-provoking narrative review of incremental HD by Murea et al, the authors highlighted the ‘pervasive unknowns’ surrounding HD dosing, particularly for patients starting chronic HD with appreciable levels of RKF. They also addressed the unclear guidelines for prescribing incremental HD. The dialysis dose in incremental HD is calculated by adding the total urea removal during dialysis to the residual renal clearance (KRU) from RKF. Unlike peritoneal dialysis (PD), where RKF and peritoneal clearance are continuous and can be easily combined, solute clearance with HD is intermittent while KRU is continuous, making it technically more complex to incorporate RKF into dialysis prescriptions. Furthermore, the decline in RKF can be highly variable and unpredictable. For example, patients may transition from pre-dialysis CKD to the early stages of chronic HD, where less frequent dialysis schedules (fewer than three times per week) may be appropriate. Conversely, they may progress to the late stages of chronic HD, where more frequent dialysis schedules (three or more times per week) are typically required.
RKF inversely correlates with dialysis vintage and can decline at a faster rate in patients with congestive heart failure, uncontrolled hypertension, proteinuria, diabetes, intradialytic hypotension and with increased HD frequency.

Kidney function before and after dialysis initiation. Residual kidney function (RKF) denotes the level of kidney function present at the time and after dialysis initiation.Figure 1 from Obi et al, Toxins June 2024, © the authors.
Several challenges exist for widespread implementation of incremental HD:
- Uncertainty regarding patient adherence and expectations related to incremental changes in HD prescriptions.
- Increased workload for nephrologists and dialysis staff, who must monitor patient parameters—such as RKF, volume status, and dialysis adequacy—more frequently and closely.
- Potential for reduced financial margins for dialysis stakeholders due to fewer overall HD treatments and lower financial reimbursements.
So, why should we consider prescribing incremental HD, and are there any potential advantages to this approach?
Regarding the first challenge, patient education prior to dialysis initiation and throughout the dialysis journey can help mitigate concerns related to patient adherence to the recommended HD schedules. Certainly, a prejudiced approach that a priori prescribes more medications or higher-level interventions based on presumed nonadherence should not influence our decision-making.
On the second challenge, automating RKF measurement and reporting, along with tracking trajectories of volume status and blood pressure control, could help alleviate the added workload for providers. Furthermore, adding and substituting the type of care delivered from HD service to monitoring RKF, along with clinical and cardiovascular parameters, would support advocacy for untruncated dialysis reimbursement even in cases of individualized HD care.
Regarding the third challenge, while incremental HD may seem to reduce financial margins, improved patient care—such as better quality of life, potentially fewer hospitalizations, and other clinical benefits—could lead to longer patient lifespans on dialysis. This, in turn, could translate into more HD treatments at outpatient dialysis centers, offsetting potential financial losses.
Dr. Golper and colleagues have proposed a fascinating theory in support of incremental HD: the intact nephron hypothesis in reverse. The intact nephron hypothesis put forward by Bricker et al in 1960 states that the nephrons that survive demise in progressive CKD increase their excretory capacity to compensate for the lost nephrons. In other words: What doesn’t kill you makes you stronger! The intact and surviving “super” nephrons undergo slow and adaptive changes to increase GFR, decrease tubular reabsorption and increase tubular secretion thereby working towards deactivating the very stimuli causing the nephron loss. It follows that initiation of dialysis may lead to a halt in the development of these adaptive changes and compromise the diseased kidney’s adaptation to nephron loss. There is evidence that HD that is delivered more often than three times per week negatively affects the trajectory of RKF based on the outcome of RKF at day 28 between participants randomized to intermittent HD three times per week and six times per week in the Renal Failure Trial Network. The study found that patients treated with more frequent HD experienced lower rates of renal function recovery by day 28. On the other hand, if dialysis is introduced gently and incrementally, the stimuli for nephron loss and the resultant adaptive changes may remain intact longer, thereby helping to preserve RKF. Thus, it has been theorized that incremental HD can preserve the “super” nephrons and help preserve RKF for longer duration. This, however, remains to be proven in RCTs.
Indeed, the decline of RKF among dialysis patients has been associated with higher mortality and poorer quality of life. The CANUSA study in PD patients highlighted that RKF is a crucial marker of patient survival, whereas the exogenous clearance provided by PD has less impact on clinical outcomes. Therefore, preserving RKF is linked to better clinical outcomes. In the context of incremental HD, the dialysis prescription can be individualized based on a patient’s RKF, with less frequent HD considered for patients who have adequate RKF and clinical parameters (such as volume status and blood pressure control), allowing for a dialysis schedule less frequent than the standard three times per week.
Despite growing evidence supporting the potential benefits of less intensive and incremental HD, the reality is that large RCT data to confirm these observations is lacking. As a step forward, several investigators have conducted trials to examine the feasibility of twice-weekly HD in incident patients starting chronic HD and larger scale RCTs to compare the effectiveness of incremental HD with that of conventional HD are being conducted.
The TwoPlus pilot clinical trial, completed in 2021, was a randomized study designed to assess the feasibility of incremental HD in the incident population. The trial demonstrated that randomization to time-delineated treatment with twice-weekly HD, combined with adjuvant pharmacologic therapy and followed by conversion to thrice-weekly HD, along with serial timed urine collection, is feasible.
Investigators further analyzed the longitudinal changes in RKF and kidney solute clearance with timed urine collections in these patients and found that despite gradual decline in RKF and solute clearance, significant kidney function persisted up to 1 year after initiation of HD.
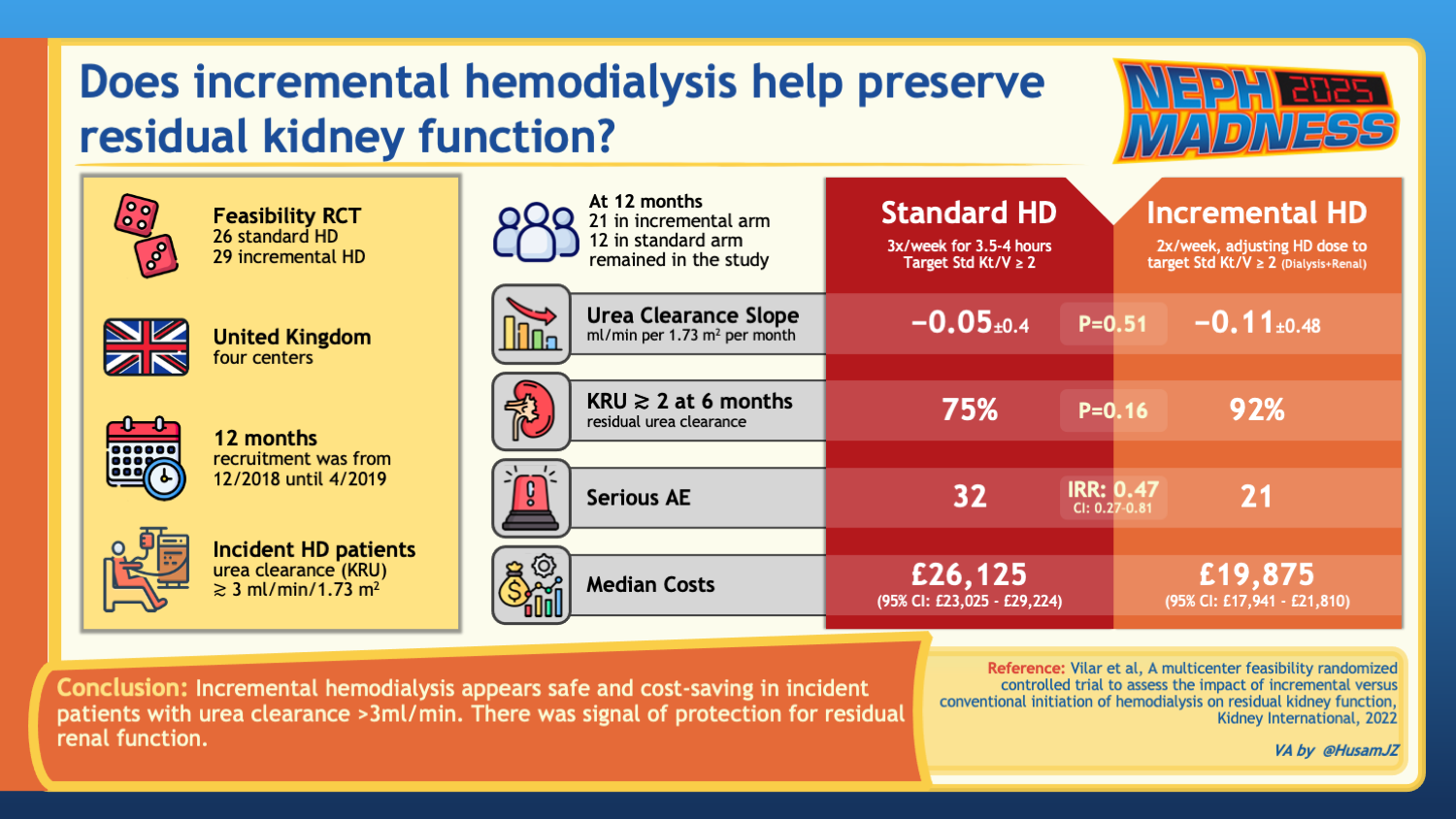
In the UK, Vilar et al. conducted a feasibility trial examining 55 incident patients with adequate RKF, comparing standard thrice-weekly HD to incremental twice-weekly HD later transitioned to thrice-weekly HD based on changes in RKF or clinical manifestations. The study found no significant differences between the groups in the rate of RKF loss at six months. However, incremental HD showed fewer serious adverse events, lower healthcare costs, and similar patient-reported outcomes compared to the standard regimen. Despite no clear benefit in RKF preservation or quality of life, the results suggest that incremental HD is safe and cost-saving, supporting the need for a definitive trial.
In another pilot RCT completed in 2024, 26 patients on HD with average KRU 4.7±1.8 ml/min and HD vintage of 12±15 months underwent a cross-over trial comparing 4 weeks of twice-weekly (goal standard Kt/V 2.2) HD and 4 weeks of thrice-weekly HD (goal spKt/V 1.3). The study found that twice-weekly HD did not impact the quality of life, while the ongoing function of residual kidneys effectively managed fluid balance, potassium levels, and uremic solutes, without requiring significantly longer treatment times.
Hitherto, three meta-analyses have been conducted by Takkavatakarn et al., Liu et al., and Garofalo et al. Collectively, these studies assessed the outcomes of incremental HD compared to conventional HD in patients treated with chronic HD. Incremental HD was associated with lower mortality and better preservation of RKF, with one analysis reporting a significant reduction in mortality (RR 0.797) and better RKF and urine volume preservation in incremental HD patients. Moreover, incremental dialysis was linked to a slower RKF decline and delayed time to full-dose dialysis, with studies showing that it may defer the need for conventional HD by approximately one year. However, the risk of mortality between incremental and conventional HD showed mixed results, with some studies suggesting no significant difference. Taken together, these findings suggest that incremental HD may offer clinical benefits, including preserving RKF, reducing hospitalizations, and lowering healthcare costs, but further large-scale RCTs are necessary to confirm these potential advantages.
As Steve Jobs said, “Simple can be harder than complex: You have to work hard to get your thinking clean to make it simple. But it’s worth it in the end because once you get there, you can move mountains.” Tailored and dynamically adjusted incremental HD prescriptions have the potential to significantly enhance patient experience and clinical outcomes.
Bringing it all together, what does it really mean to have a patient- or person-centered approach in patients on chronic dialysis? We should aim towards a future in which each patient’s dialysis prescription should be based on their RKF, underlying comorbidities, and quality of life, while allowing fluidity in prescription changes based on these parameters and not just the objective measures like Kt/V, phosphorus, PTH, and hemoglobin levels. With various modalities and interventions available, the dialysis therapy could be molded to individual patient needs–whether it is the PD-first approach or HDF or incremental HD or conventional HD. We need to meet the patients where they are by addressing individual goals and challenges to support them on their enduring road on dialysis.
In conclusion, the current landscape of chronic dialysis features a growing interest in two key approaches: HDF and incremental HD. Both interventions show promise in enhancing clinical outcomes and improving the quality of life for patients receiving chronic dialysis.
COMMENTARY BY MARIANA MUREA:
The Future of Dialysis Personalization
– Executive Team Members for this region: Jeffrey Kott @jrkott27 – @jrkott27.bsky.social and Dia Waguespack @d_r_waguespack | Meet the Gamemakers
How to Claim CME and MOC
US-based physicians can earn 1.0 CME credit and 1.0 MOC per region through NKF PERC (detailed instructions here). The CME and MOC activity will expire on June 1, 2025.






Leave a Reply