Stiff Arteries, Fragile Kidneys: The Link to Chronic Kidney Disease
Dr Api Chewcharat is currently a clinical and research fellow at Brigham and Women’s hospital (BWH)/Massachusetts General Hospital (MGH) Nephrology combined program. His hometown is Bangkok, Thailand. He earned a Master of Public Health in Biostatistics and Epidemiology from Harvard T.H. Chan School of public health before completing his internal medicine residency at Mount Auburn Hospital/Harvard medical school. He has recently been selected to receive grant support from the American Kidney Fund Clinical Scientist in Nephrology Award. His research focuses on onconephrology and causal inference. Outside of medicine, he enjoys swimming, biking, playing video games and cooking. Follow him @Api_chew. Dr Chewcharat is a 2024-25 AJKD Editorial Intern.
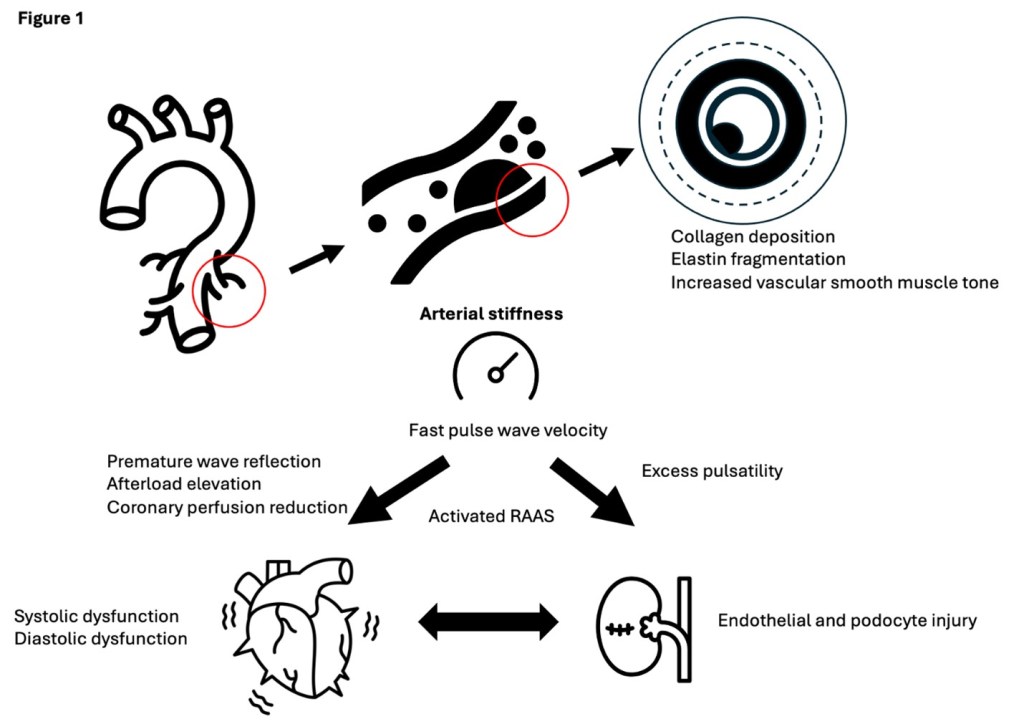
Arterial stiffness is characterized by a diminished elasticity of the arterial wall, typically measured by pulse wave velocity (PWV). Mechanistically, collagen deposition, elastin fragmentation and increased vascular smooth muscle tone progressively stiffen the vessel. This process is commonly seen in hypertension, diabetes and chronic inflammation. As arterial stiffness worsens, the pressure waves generated during systole are transmitted more forcefully through the vascular tree, subjecting the delicate renal microcirculation, especially glomerular capillaries, to greater mechanical stress. This hemodynamic burden promotes endothelial and podocyte injury, accelerates nephron loss, and undermines the kidney’s autoregulatory capacity. Moreover, stiffening of the arteries triggers neurohormonal cascades, particularly through the renin-angiotensin-aldosterone system (RAAS). The resulting afterload elevation can also lead to left ventricular hypertrophy and subsequent systolic or diastolic dysfunction, both of which further predispose the kidneys to hypoperfusion and ischemic injury (Figure 1).
In a recently published AJKD article “Arterial Stiffness and Subsequent Incidence of CKD and Kidney Function Decline in a Large Longitudinal Community Cohort: The Atherosclerosis in Communities (ARIC) Study” by Yao et al., the authors leveraged data from the Atherosclerosis Risk in Communities (ARIC) study. Patients were followed up for a median of 6.6 years. The authors found an association between increased arterial stiffness, particularly carotid-femoral PWV, and risk of both incident chronic kidney disease (CKD) [hazard ratio 1.53, (95% CI, 1.15-2.04)] and rapid kidney function decline (-0.44 ml/min/1.73 m2/year [95% CI, -0.56 to -0.33]). These findings highlighted that arterial stiffness is not merely a cardiovascular risk factor, but also an important factor in the pathogenesis of CKD.
Another important aspect of arterial stiffness, especially in older or high-risk patients, is medial arterial calcification. Unlike atherosclerotic intimal calcification, medial calcification is associated with age, diabetes, and CKD, and serves as a robust predictor of cardiovascular mortality. This type of calcification can worsen arterial stiffness and kidney function decline, and conversely, advanced CKD amplifies the propensity for medial calcification, illustrating a bidirectional relationship. Investigations into therapies that mitigate medial calcification, such as vitamin K and magnesium supplementation remain controversial. Although Yao et al. did not specifically address the mechanisms underlying arterial stiffness, future research should focus on pathophysiological mechanisms underlying medial calcification to make way for the development of novel preventative or therapeutic strategies.
Given the substantial role arterial stiffness appears to play in both cardiovascular and renal disease, these findings underscore the importance of routine screening and potential interventional strategies, particularly among older adults or individuals with metabolic syndrome. Early identification of high PWV might enable more aggressive management of blood pressure, inflammation, and metabolic derangements, ultimately slowing kidney function decline. Statins, traditionally prescribed for hypercholesterolemia and atherosclerotic cardiovascular disease prevention, have shown beneficial impacts on arterial stiffness through improved endothelial function, reduction in inflammation, and stabilization of vascular plaques. Expanding statin usage to individuals with elevated PWV who do not meet classical cholesterol-related or ASCVD risk indications could offer both cardiovascular and renal protective effects. Similarly, sodium glucose cotransporter-2 inhibitors (SGLT-2i), already widely recognized for their cardio- and reno-protective effects, could represent another therapeutic indication. Emerging research indicates that SGLT-2i may help reduce arterial stiffness by improving endothelial function, lowering blood pressure, and optimizing volume status. Considering their favorable safety profile and multifaceted benefits, SGLT-2i could be considered a logical option for patients identified as having notably high arterial stiffness. In addition, investigational medications such as fimasartan, a novel angiotensin receptor blocker (NCT02022774), and supplementation with equol, a metabolite of soy isoflavone (NCT05741060), are currently being explored for their potential effects on arterial stiffness.
In light of these insights, further studies are warranted to investigate whether targeted treatment of arterial stiffness can effectively postpone CKD onset or decelerate its progression in at-risk populations. Additionally, cost-effectiveness analyses of widespread arterial stiffness screening among high-risk groups such as elderly patients with metabolic syndrome would shed light on the feasibility of implementing such strategies at scale.
-Post prepared by Api Chewcharat
To view Yao et al (Open Access), please visit AJKD.org:
Title: Arterial Stiffness and Subsequent Incidence of CKD and Kidney Function Decline in a Large Longitudinal Community Cohort: The Atherosclerosis in Communities (ARIC) Study
Authors: Zhiqi Yao, Junichi Ishigami, Esther Kim, Shoshana H. Ballew, Yingying Sang, Hirofumi Tanaka, Michelle L. Meyer, Josef Coresh, and Kunihiro Matsushita
DOI: 10.1053/j.ajkd.2024.11.011

Leave a Reply