Atlas: Clinical Quiz 4
Post prepared by and all images courtesy of Tibor Nadasdy, MD, AJKD Blog Contributor and AJKD Kidney Biopsy Teaching Case Advisory Board member.
For a PDF version of the question & answer, please click here.
Click on an image for a larger view.
A 41-year-old morbidly obese (weight 283 pounds, height 5 feet 4 inches) woman presented with 1 week of bilateral lower extremity swelling and an 8-lb weight gain. She is not diabetic, but has a history of well controlled hypertension. She has been taking NSAID regularly for joint pains. She reports an episode of pharyngitis that was treated with antibiotics three weeks earlier. Three months ago, her serum creatinine was 1.0 mg/dL (eGFR, 70 mL/min/1.73m2). At presentation, her serum creatinine was elevated to 2.3 mg/dL (eGFR, 26 mL/min/1.73m2). A spot urine protein/creatinine ratio was 13.9 g/g. Urinalysis detected numerous dysmorphic red blood cells. Her C3 was low at 47 g/L, and C4 was normal at 30 g/L. Antistreptolysin O titer was mildly elevated at 216. Other serologies, including ANA, ANCA, hepatitis B and C, and HIV, were negative. Her blood pressure was 160/90 mmHg. A kidney biopsy was performed. What is the most likely diagnosis?
C3 glomerulopathy
Incorrect.
Staphylococcus infection–associated glomerulonephritis
Incorrect.
Poststreptococcal glomerulonephritis
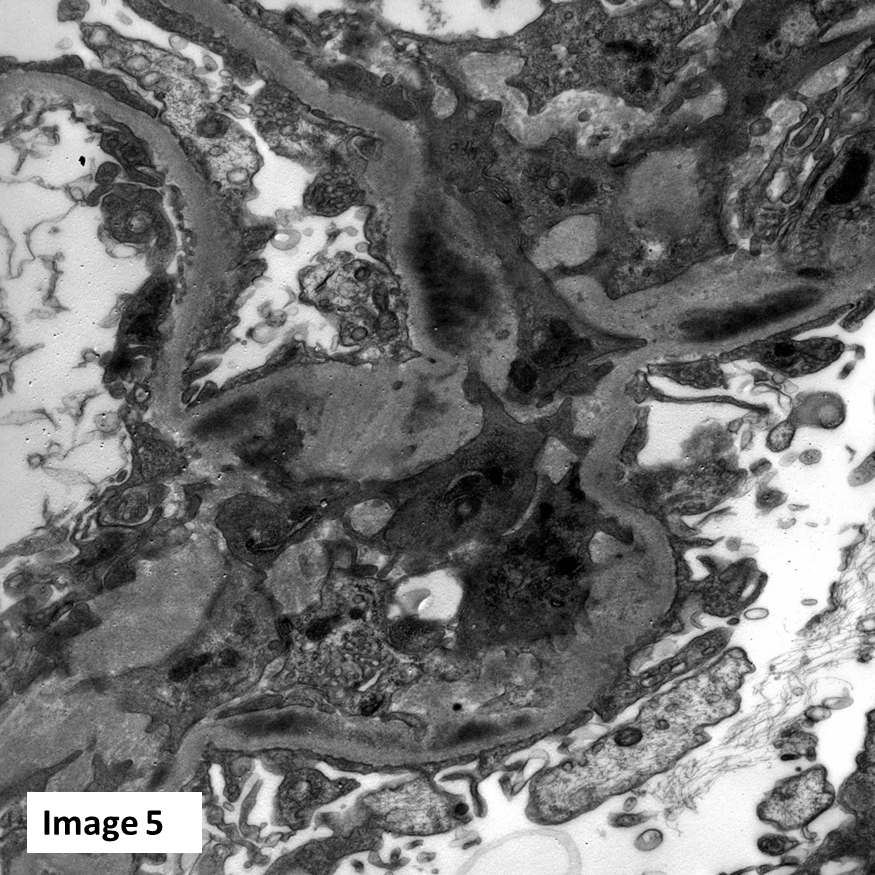
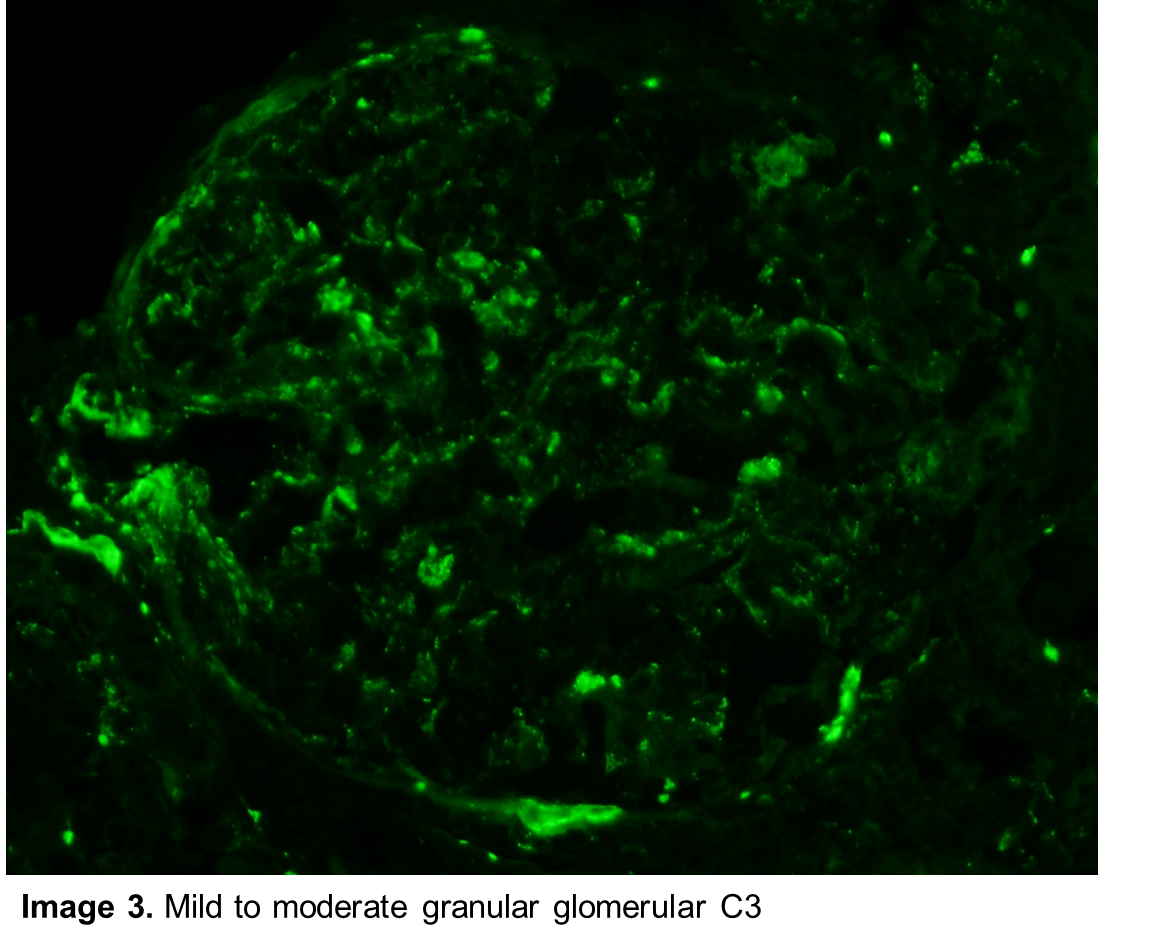
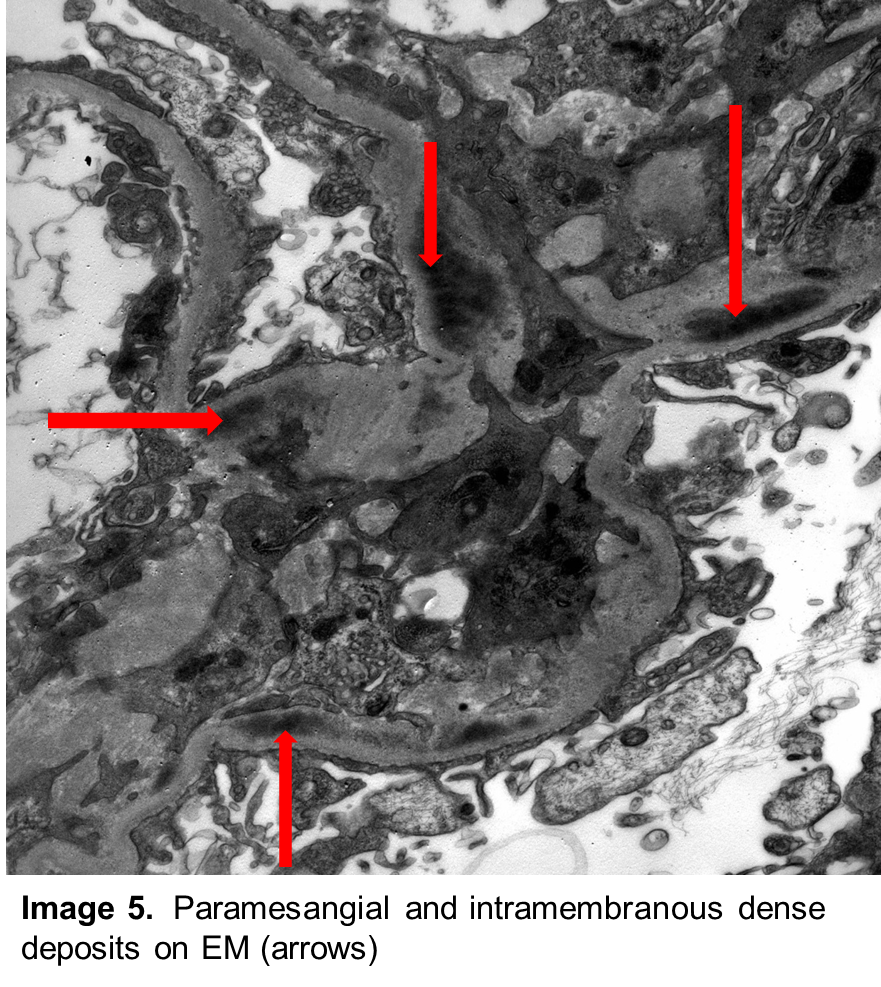
Correct! Paraffin sections contained renal cortex with 13 glomeruli; only one was globally sclerotic. The glomeruli showed diffuse global intracapillary hypercellularity (Image 1) with occasional neutrophils (Image 2). There was evidence of acute tubular injury with some tubular epithelial irregularity. Only mild patchy interstitial inflammatory cell infiltrate was noted. Interstitial fibrosis and tubular atrophy were mild, involving approximately 5% of the renal cortex. Frozen sections for immunofluorescence contained 15 glomeruli. There was granular mesangial and scattered glomerular capillary fluorescence for C3 (Image 3). No glomerular staining was seen with antibodies to IgG, IgA, IgM, kappa and lambda light chains, C1q, and fibrinogen. Ultrastructural examination revealed scattered large subepithelial “humps” along the glomerular capillary loops (Image 4). Occasional intramembranous and mesangial deposits were also noted (Image 5). Podocyte foot process effacement was widespread.
The patient was treated with conservative renoprotective measures, and NSAIDs were discontinued. No immunosuppressive medications were given. Two weeks later the patient’s serum creatinine decreased to 1.72 mg/dL (eGFR, 36 mL/min/1.73m2) and her edema decreased substantially.
Nowadays, we rarely see poststreptococcal glomerulonephritis in kidney biopsy material, particularly in adults. The differential diagnosis is primarily C3 glomerulonephritis/glomerulopathy and glomerulonephritis associated with nonstreptococcal infections. Heavy nephrotic proteinuria is unusual in poststreptococcal glomerulonephritis; in this patient, morbid obesity and, perhaps NSAID use, may have aggravated the proteinuria. Other infectious agents may cause similar histology, but the history of pharyngitis three weeks before presentation, the C3-dominant glomerular subepithelial deposits (“humps”), the low serum C3 level, the slightly elevated ASO titer, and the improvement in kidney function and proteinuria without steroid treatment all favor of poststreptococcal glomerulonephritis.
C3 glomerulonephritis is a relatively recently described entity and bears many similarities to postinfectious/poststreptococcal glomerulonephritis. In fact, the differential diagnosis can be quite difficult because patients with C3 glomerulopathy frequently have low serum C3 levels and may even have subepithelial “humps.” There is some evidence that, occasionally, infections, including streptococcal infections, can initiate the development of C3 glomerulonephritis. In such cases, the glomerular disease starts as a postinfectious glomerulonephritis, but unlike postinfectious glomerulonephritis, it will not resolve and becomes a chronic, persistent, slowly progressive form of glomerulonephritis. IgG deposits are absent or very mild in C3 glomerulopathy. In poststreptococcal glomerulonephritis, IgG deposits may or may not be present. In our experience, it is common to see C3-dominant or only C3-positive glomerular capillary deposits in poststreptococcal glomerulonephritis, making the differential diagnosis even more difficult. Also, IgG deposits may be masked and can only be revealed following antigen retrieval (protease digestion of the paraffin sections). If any doubt, we and others recommend performing immunofluorescence for immunoglobulins following protease digestion of paraffin sections, which can unmask the IgG in the deposits. It is important to differentiate C3 glomerulopathy from poststreptococcal or infection-associated glomerulonephritis because of the very different treatment approaches and outcomes. If an apparent poststreptococcal glomerulonephritis does not achieve remission, C3 glomerulonephritis (or if IgG is present, membranoproliferative glomerulonephritis) should be considered. The best method diagnosing C3 glomerulopathy is testing for alternate pathway complement regulatory abnormalities, including genetic abnormalities in alternate pathway regulatory proteins or autoantibodies to them, such as C3 nephritic factor.
While poststreptococcal glomerulonephritis became a rare diagnosis in the everyday renal pathology practice, the number of cases with staphylococcus infection–associated glomerulonephritis is on the rise. In staphylococcus-associated glomerulonephritis, the staphylococcus infection is frequently still ongoing; therefore, the term postinfectious glomerulonephritis should not be used. Patients with staphylococcus infection–associated glomerulonephritis usually have numerous underlying comorbidities such as diabetes. Serum complement levels are low in less than 50% of patients with staphylococcus infection–associated glomerulonephritis, while they are low in approximately 90% of patients with poststreptococcal glomerulonephritis. The kidney biopsy findings can be similar, but in staphylococcus infection–associated glomerulonephritis, the glomerular deposits usually (but not always) contain IgA, in addition to C3. Large subepithelial deposits (humps) occur in approximately 30 percent of biopsies with staphylococcus infection–associated glomerulonephritis; they are practically always seen in active poststreptococcal glomerulonephritis. Obviously, the safest differential diagnostic tool is to identify staphylococcus as the infectious agent, which, unfortunately, can be difficult. Blood cultures may be negative, and sometimes the site of infection is not obvious. If there is cellulitis or an infected wound, cultures from the infected site should be performed.
There are morphologic overlaps between membranoproliferative glomerulonephritis and poststreptococcal glomerulonephritis, which can make the diagnosis difficult. Occasionally, humps can occur but, in a typical case of membranoproliferative glomerulonephritis, the glomerular capillary deposits are mainly subendothelial and there is concomitant IgG staining. Also, membranoproliferative glomerulonephritis is not a self-limiting disease, unlike poststreptococcal glomerulonephritis. A glomerulonephritis with membranoproliferative pattern without IgG deposits now should be classified as C3 glomerulonephritis/C3 glomerulopathy (see above). In this patient, the absence of IgG in the glomerular capillary deposits, the numerous humps, the absence of subendothelial deposits, and the fast recovery without steroid treatment make the diagnosis of membranoproliferative glomerulonephritis unlikely.
Membranoproliferative glomerulonephritis
Incorrect.
Please visit the Atlas of Renal Pathology II at AJKD.org to view related installments (freely available).








Thanks a lot