Gout and CKD: Harmonizing Urate Control with Patient-Centric Care
Dr. Suzanne El-Sayegh serves as the Program Director of the Internal Medicine Residency at Staten Island University Hospital, part of the Northwell Health System. In this pivotal role, she oversees the training and development of Internal Medicine residents and fellows, demonstrating her commitment to fostering the next generation of healthcare professionals. In addition, she serves as the Director of Nephrology and Associate Chair of Medicine. She is an accomplished educator and clinician who holds the title of Professor at the Donald and Barbara Zucker School of Medicine, with a strong track record of presenting at local, regional, and international medical conferences.
Hachem Araji (@ArajiHachem) is a second-year internal medicine resident at Staten Island University Hospital-Northwell Health in New York. He graduated with a Doctor of Medicine from Lebanese American University in Lebanon. His research experience includes work on organ donation and transplantation. With a passion for nephrology and a specific focus on transplant nephrology, he is poised to make significant contributions to the field of medicine.
Nadim Zaidan is currently an internal medicine resident at the Northwell Staten Island University Hospital. He graduated from the Saint Joseph University Faculty of Medicine in Beirut in 2021. He is interested in all aspects of nephrology and is looking forward to applying for a Fellowship in 2025.
Patients with chronic kidney disease (CKD) have an impaired ability to clear uric acid from plasma. The accumulation of uric acid (UA) in CKD results in asymptomatic hyperuricemia, which has been implicated as a potential risk factor for CKD progression and hypertension. This association is primarily supported by evidence derived from experimental animal models. On an epidemiological level, data from the NHANES cohort suggests that approximately two-thirds of patients diagnosed with gout had at least CKD II or worse; more impressively, 1 in 5 gout patients had CKD IV.
Persistent elevation of serum uric acid concentrations beyond physiological thresholds can precipitate a cascade of events culminating in gout. This time-dependent process of crystal formation and deposition underscores the importance of long-term urate-lowering strategies in the management and prevention of gout, particularly in individuals with chronic hyperuricemia.
Allopurinol and febuxostat are two xanthine oxidase (XO) inhibitors, which by blocking hypoxanthine conversion, reduce the production of uric acid. The therapeutic objective for urate-lowering agents, as recommended by multiple rheumatology societies and clinical guidelines, is to reduce serum uric acid concentrations below a specific threshold. This target is typically set at <6 mg/dL, although in cases of severe gout or tophaceous deposits, a more stringent goal of <5 mg/dL may be advised. These targets are established with consideration of the physiochemical properties of uric acid, specifically its solubility limit of approximately 6.8 mg/dL in physiological conditions.
In a post-hoc analysis of the STOP Gout Trial, a treat-to-target, randomized controlled trial, allopurinol and febuxostat were compared head-to-head for 72 weeks, divided in a triphasic period, to assess their ability to reduce gout flares and bring uric acid levels down to target while monitoring for adverse events. This clinical trial recruited CKD patients and employed a dynamic design that closely mimics real-world practice by allowing dose adjustments throughout the follow-up period. This approach contrasts with previous studies that used fixed doses or lacked adequate representation of CKD patients, a population disproportionately affected by hyperuricemia. The study is particularly relevant for CKD patients, whose fluctuating renal function often necessitates ongoing medication adjustments to balance efficacy and safety. Longitudinal data from this extended study demonstrated equivalent efficacy and safety profiles for both xanthine oxidase inhibitors when employing a treat-to-target approach in stage III CKD patients.

Mean eGFR over all trial phases. Mean eGFR slopes were 1.2mL/min/1.73m2 per year among allopurinol-treated patients and 0.8mL/min/1.73m2 per year among febuxostat-treated patients (P=0.73). Abbreviation: eGFR, estimated glomerular filtration rate. Figure 1 from Helget et al, © National Kidney Foundation.
These results come at a time when uric acid lowering therapy mainly focuses on the prevention of CKD progression. While initial prospective cohort studies have suggested that initiation of allopurinol at a minimum of 300 mg in patients with gout is associated with a reduced rate of kidney function deterioration, results of randomized clinical trials have been more controversial. Indeed, the PERL and CKD-FIX did not identify any decrease in CKD progression rate with allopurinol-reduced hyperuricemia. While both the PERL– and CKD-FIX– placebo-controlled trials found no significant difference in cardiovascular events with allopurinol use, the CARES trial revealed an unexpected and significant increase in cardiovascular mortality with febuxostat compared to allopurinol. It is worth noting that these safety issues were not identified in the STOP Gout and FAST trial where neither XO inhibitors were reported to increase cardiovascular risk and were not associated with higher overall mortality.
The STOP Gout Trial’s dynamic dose adjustment protocol offered a significant methodological advantage. This approach not only provided treatment flexibility but also yielded important empirical data. Notably, it demonstrated that patients with stage III CKD required mean doses of 395 mg for allopurinol and 64 mg for febuxostat to achieve target urate levels. These dosages were remarkably similar to those typically used in the general population, suggesting that moderate renal impairment may not substantially alter the dose-response relationship for these urate-lowering therapies. This finding has important implications for optimizing treatment strategies in CKD patients and potentially broadens the applicability of standard dosing regimens. Interestingly, data suggest that achieving equivalent serum urate control to target levels necessitated more frequent dose titrations for allopurinol compared to febuxostat. Goal achievement was similar between both groups and expectedly patients who did not meet the target were more prone to experience flares in the last phase. This trial, however, focused on patients with stage III CKD, limiting applicability to those with more severe CKD.
Although both drugs had a similar level of uric acid target achievement, allopurinol was associated with a lower rate of gout flares during all three phases of the study compared with febuxostat (p <0.001). This treatment-related reduction in flares did not hold in sensitivity analyses after accounting for the difference in attrition rate between both treatment groups.
Adverse events with these medications at eGFRs lower than 60mL/min have constantly been a major concern with poor evidence guiding their ideal prescription dose and the optimal up-titration regimen. Slow-up titration is not only an adequate approach to identify the optimal dose for each patient, but it also allows for risk mitigation when it comes to adverse events, notably of severe allergic reactions like Allopurinol Hypersensitivity Syndrome or acute kidney injury, which in this study was found to be predominantly pre-renal. Managing gout in CKD is difficult due to limited evidence and concerns about medication safety and efficacy, leading to inconsistent and often inadequate treatment. By shifting the focus on symptom control, the emphasis is placed on quality of life, which is essential in a group of patients known to have a higher pain burden than the general population (Table).
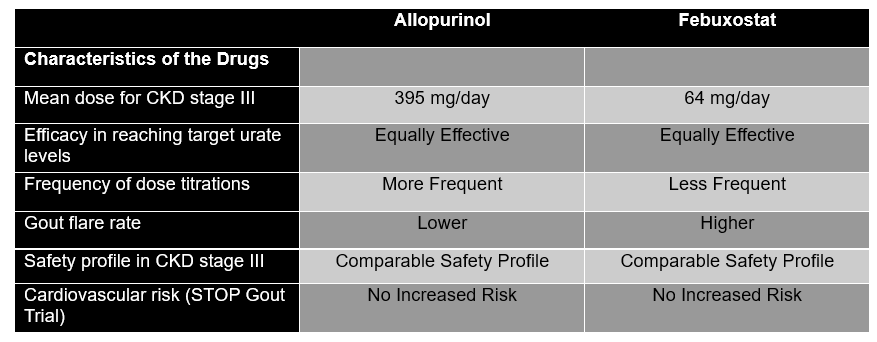
Table. Key findings from the STOP Gout Trial Comparing Allopurinol to Febuxostat © El-Sayegh, Araji, Zaidan.
In conclusion, addressing hyperuricemia in CKD patients, while not necessarily associated with decreased CKD progression, has the potential to reduce gout flares thus improving quality of life and potentially avoiding unnecessary costs related to health care services during acute flares. Ultimately, given the chronic nature of gout and its frequent co-occurrence as kidney disease progresses, reduction in morbidity using evidence-based, safe, and effective therapeutic agents like XO inhibitors is a major advance for patients with CKD. The key remains to individualize treatment based on CKD stage, comorbidities, and gout severity while carefully weighing risks and benefits. Future studies should include more CKD patients and report outcomes stratified by renal function. The safety profile of urate-lowering therapy appears acceptable, but more research is needed in patients with more severe renal impairment.
– Post prepared by Dr. Suzanne El-Sayegh, Hachem Araji @ArajiHachem, and Nadim Zaidan
To view Helget et al (subscription required), please visit AJKD.org:
Title: Efficacy and Safety of Allopurinol and Febuxostat in Patients With Gout and CKD: Subgroup Analysis of the STOP Gout Trial
Authors: Lindsay N. Helget, Anne Davis-Karim, James R. O’Dell, Ted R. Mikuls, Jeff A. Newcomb, Maria Androsenko, Mary T. Brophy, Bryant R. England, Ryan Ferguson, Michael H. Pillinger, Tuhina Neogi, Hongsheng Wu, and Paul M. Palevsky
DOI: 10.1053/j.ajkd.2024.04.017


Leave a Reply