Addressing Anemia in CKD and ESRD: Will Hypoxia-Inducible Factor Stabilization Eliminate the Need for Erythropoietin Stimulating Agents?
Diana Mahbod, MD, CPE, FASN, FNKF, is a nephrologist in the Dallas-Fort Worth metroplex. She completed a Nephrology fellowship at University of California, San Francisco (UCSF) and has worked as a private practice nephrologist since 2018, most recently transitioning to telenephrology to provide care to patients in underserved areas of the country. Dr. Mahbod (@DiMiRenalMD) is enthusiastic about education and mentorship and is a graduate of the GlomCon Glomerular Disease Fellowship (2020) and Kidney Pathology Certificate Program (2024), and is a medical content editor for McGraw Hill, and a mentor to trainees in the NephSim program.
As nephrologists, we know that the role of the kidneys is to maintain hemostasis in the body. We counsel patients about the role of the kidneys in clearing toxins, maintaining an optimal electrolyte and acid-base equilibrium, and balancing salt and water balance. We also discuss the role of the kidneys in helping to regulate blood cell production via release of erythropoietin (EPO). Providing erythropoietin-stimulating agents (ESAs) is a mainstay of nephrology practice, but as with any system in the body, maintenance of optimal blood cell counts involves a complex and multifaceted series of requirements within the body. Deficiencies in nutrients such as iron, vitamin B12, and folate, as well as alternative causes of anemia such as infection and inflammation, must be considered and treated.
In recent years, hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHI) have been studied as alternative agents to ESAs. HIF is a transcription factor (composed of HIF-alpha and HIF-beta dimers) that activates multiple target genes associated with iron metabolism and erythropoiesis. The action of HIF has been shown to improve anemia even when blood EPO concentrations are low and even when chronic inflammatory conditions are high (based on high CRP levels).
In normal conditions, hypoxia-inducible factor prolyl hydroxylase (HIF-PH) is active and continuously rapidly degrading HIF. Inhibition of HIF-PH allows for the HIF dimers to bind and excerpt their actions, including stimulating EPO production and positively impacting transferrin and iron binding capacity (see figure).

Mechanism of action of HIF stabilizers. Under normal conditions, the activity of HIF-PH leads to the rapid degradation of HIF. During hypoxia, HIF-PH activity is instead suppressed, resulting in stimulation of endogenous EPO production, increased expression of the transferrin receptor, better use of iron with consequent maturation of erythrocytes, and an increase in hemoglobin levels. Similarly, the use of HIF stabilizers results in a steady increase in dose-dependent hemoglobin levels, including reducing hepcidin and ferritin levels and improving iron binding capacity. Figure 1 from Crugliano et al, © 2021 by the authors.
Compared to ESAs, HIF-PHIs (which stabilize HIF, allowing for beneficial effects) may have some advantages. ESAs are administered parenterally and require cold storage, whereas HIF-PHIs are taken orally. Additionally, due to the potential for increase in risk of cardiovascular events with higher doses of ESAs, the goal of treatment with an ESA is not to normalize the hemoglobin level, but to increase it to 9-11 grams/deciliter. Further investigation is needed to determine if use of HIF-PHIs to increase endogenous production of EPO and improve iron availability would have a more favorable risk profile.
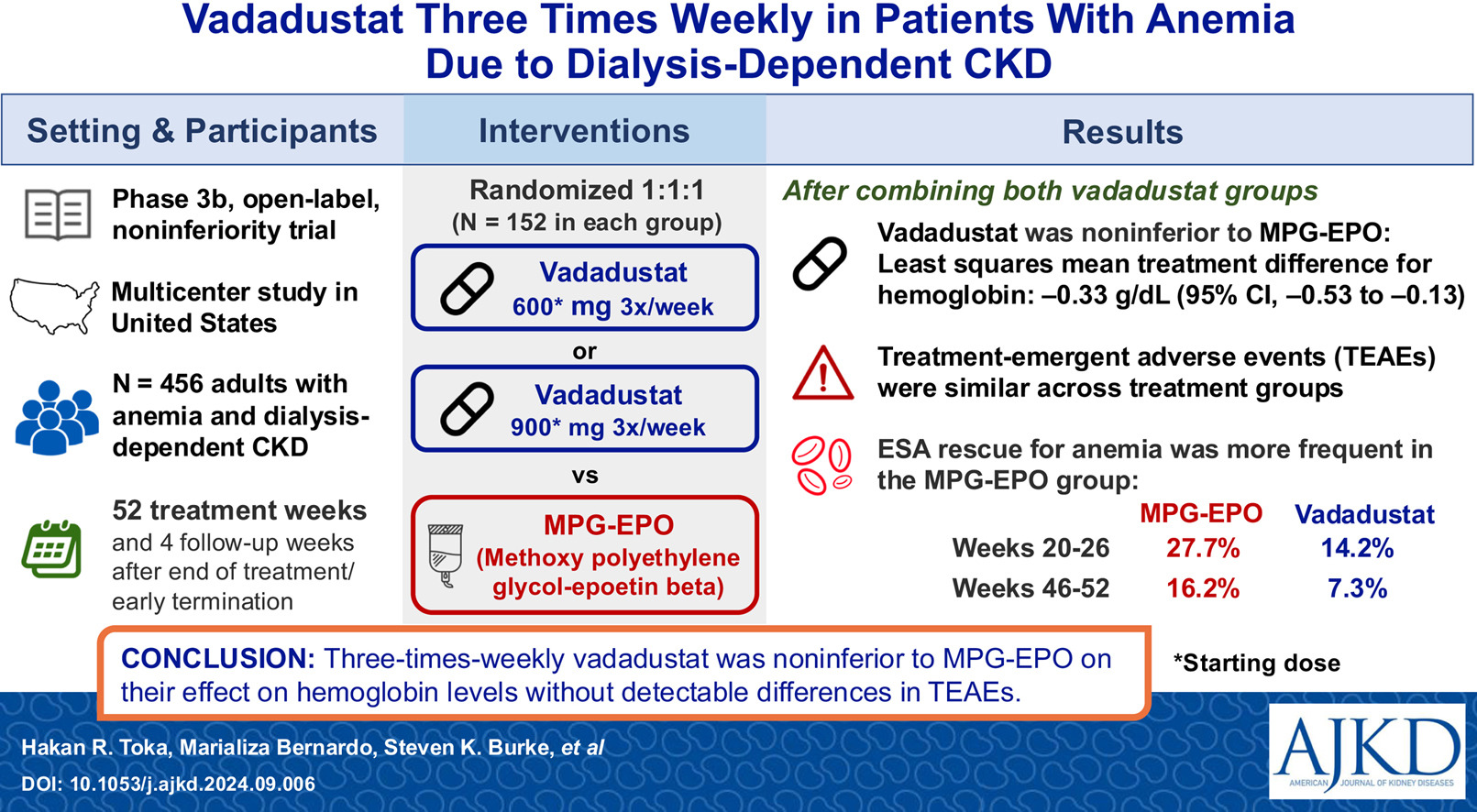
In the FO2CUS trial, Toka et al. studied vadadustat (an HIF-PHI) in comparison to methoxy polyethylene glycol-epoetin beta (MPG-EPO, a long-acting ESA) in a phase 3b non-inferiority trial. Study participants received a lower dose of vadadustat (600mg thrice weekly), higher dose vadadustat (900mg thrice weekly), or MPG-EPO for up to 52 treatment weeks, with 4 safety follow-up weeks after the end of treatment or early termination from the trial. In a previous trial (INNO2VATE), vadadustat (dosed initially at 300mg once daily, dose adjusted to a maximum of 600mg daily if needed) was non-inferior to darbepoetin alfa (another ESA) with similar overall safety profiles. To align with dialysis schedules for convenience, Toka and colleagues opted for thrice weekly dosing and compared it to MPG-EPO.
The trial was a multi-center, randomized trial conducted in the United States from 2021-2023. Patients were adults receiving thrice-weekly in-center hemodialysis and had mean hemoglobin levels of 8.5-11 grams/deciliter and normal iron levels during screening. Patients were required to have been actively receiving MPG-EPO prior to the screening visits. Patients who had received darbepoetin alpha within the month prior to screening, and those with bleeding events, RBC transfusion in the 2 months prior to screening, or with known causes of anemia other than CKD, were excluded. Once enrolled, patients received either vadadustat 600mg or 900mg thrice weekly, or MPG-EPO according to the dialysis unit protocol. Doses were adjusted to maintain hemoglobin levels between 10-11 grams/deciliter. Of note, vadadustat dose could have been adjusted to as low as 300 milligrams or as high as 1200 milligrams thrice weekly. In both vadadustat groups, “rescue” treatments with ESA or RBC transfusion were allowed when medically necessary.
Patients tolerated the trial well, with a 94% treatment compliance rate among all treatment groups. Researchers defined the primary and secondary efficacy endpoints to be mean change in hemoglobin during a primary evaluation period (weeks 20-26) and a secondary evaluation period (weeks 46-52) and found that mean change in hemoglobin concentrations over time were non-inferior to MPG-EPO, though a higher proportion of patients receiving vadadustat were below the target hemoglobin range (10-11 grams/deciliter). In terms of safety, treatment-emergent adverse effects (TEAEs) were higher in the vadadustat groups than in the MPG-EPO group, most commonly gastrointestinal side effects including diarrhea. Serious TEAEs and mortality were similar between groups (most frequently infections), except for cardiac arrests which were higher in the MPG-EPO group (7.3%) than in the vadadustat total group (1.7%). Overall, almost half of patients in the vadadustat 600mg thrice weekly group required titration to 900mg or 1200mg, suggesting that the 600mg dose may be too low for some patients (though 18% titrated down to 300mg during the trial).
Researchers concluded that thrice-weekly vadadustat was non-inferior in efficacy to a long-acting ESA, was generally well-tolerated despite a mild increase in gastrointestinal side effects, and demonstrated a similar overall safety profile to MPG-EPO, with a lower incidence of cardiovascular events compared to the ESA. Novel HIF-PHIs require long-term follow-up to determine if safety issues will arise subsequent to the one-year trial period assessed in trials like INNO2VATE and FO2CUS. Adoption of novel therapies into clinical practice has the potential to benefit patients in terms of efficacy and reduction in side effects, but economics and logistics will often play a role. It is crucial for clinicians to become aware of how decisions are made regarding the use of novel therapeutics in our practices, and the dialysis unit is an example of a setting where this is particularly important, as we are the advocates for our patients’ safety and well-being. It is also important to consider that a patient who is admitted to the hospital may not have access to the new agents, so the clinician must decide whether to use an ESA in that setting, or to withhold this aspect of anemia treatment until the patient returns to their outpatient unit. Despite the uncertainty when considering new treatment options, investigation into novel treatments for anemia secondary to kidney disease is one of many recent innovations within the field of nephrology, making it an exciting time to care for patients, as we strive for improvements in quality of life and clinical outcomes.
-Post prepared by Diana Mahbod
To view Toka et al (Open Access), please visit AJKD.org:
Title: Vadadustat Three Times Weekly in Patients With Anemia Due to Dialysis-Dependent CKD
Authors: Hakan R. Toka, Marializa Bernardo, Steven K. Burke, Wenli Luo, Roberto Manllo-Karim, Irfan Ullah, Zhihui Yang, Zhiqun Zhang, James Tumlin
DOI: 10.1053/j.ajkd.2024.09.006

Leave a Reply