#NephMadness 2019: Hypertension Region
Submit your picks! | NephMadness 2019 | #NephMadness | #HTNRegion
Selection Committee Member: Susan Steigerwalt
Susan Steigerwalt has been a hypertension specialist and nephrologist since 1986. She is a clinical Associate Professor at the University of Michigan. Her current interests include efficacy testing of a recently developed interactive app for the DASH diet, as well as advocating for improved Medicare for All- HB 1384. Follow her @SteigSusan.
Writer: Swapnil Hiremath @hswapnil
Swapnil Hiremath is a Staff Nephrologist at the Ottawa Hospital, an Associate Professor in the Faculty of Medicine at the University of Ottawa. He is a co-founder of #NephJC and #NSMC faculty. His highest rank in NephMadness was second place in 2014. His proudest accomplishment is being the originator of the #BlueRibbonFail hashtag.
Competitors for the Hypertension Region
EU Guidelines vs US Guidelines
Hyperaldo Diagnosis vs Hyperaldo Treatment

Copyright: JPC-PROD / Shutterstock
The Battle of the Guidelines
The battle of competing guidelines returns to NephMadness. After the Hyponatremia Region in 2018, we now take on hypertension in 2019. In this case, there is truly a lot of tension. And there’s more than just the Atlantic Ocean dividing the dramatically different opinions across the two continents; it’s the definition of hypertension itself! For the Europeans, hypertension begins at 140/90 mm Hg, but if a person with 135/86 in Berlin goes to Washington DC, they will now be called ‘hypertensive’, as the definition of hypertension is 130/80 in the US. Differences that one cannot just SPRINT across or jump over. Both guidelines, freely available, are scholarly works covering the evidence over many pages, numerous tables, and thousands of references. In our review, we will restrict our scope to a few choice aspects; however, both guidelines are worth reading and reflecting upon for the interested hypertension enthusiast, ie every nephrologist.

EU Guidelines

Copyright: KateChe / Shutterstock
Workgroup, Evidence Review, and Organization
The European Guidelines were produced by a workgroup on behalf of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH), with the task force members comprising 28 non-nephrologists, and no formal input from the European Renal Association. The ESH/ESC has been at it for a few years, with guidelines being produced in 2003, 2007, and 2013 prior to this update. The EU Guidelines were published simultaneously in the European Heart Journal and the Journal of Hypertension, but are also easily available on their website, and also from their app. They use 3 numbered classes of recommendations and 3 alphabetical levels of evidence, as summarized below:
Class of Recommendations ranges from ‘class I’, which is generally agreed upon and recommended/indicated, to ‘class II’, subdivided into IIa (should be considered) and IIb (may be considered). Last is ‘class III’ where there is general agreement that a given treatment of procedure is not useful or may be harmful.
Level of Evidence ranges from A (multiple trials or meta-analyses) to B (single trial or large observational studies) and C (consensus of opinion or small studies). This can be summarized as follows:
- IA = strong recommendation to do something
- IIIA = strong recommendation to not do something
- IIb C = expert opinion that something may be considered (weakest combination of recommendation and evidence)
The guideline document is well-organized, with sections in the beginning on what is new and changed from 2013, new sections, plenty of tables (33) and boxes (too many to count), and a summary of 12 key messages at the end. All the references (629) are organized at the end of the manuscript. The workgroup did not perform their own systematic review of the literature as many good reviews already existed.
BP Measurement
The table below shows the different methods of BP measurement, with their advantages and caveats:
| Method | Details | Advantages | Caveats |
| Office Manual Casual | One reading taken as patient walks in to clinic/office | Easy, available universally | Least accurate |
| Office Manual Resting | Proper BP measured after adequate rest, using correct methods | Standard method | Requires experienced staff
Rarely performed outside specialized hypertension centers or clinical trials |
| Office Automated Oscillometric BP (AOBP) | Oscillometric (electronic) method
Many devices allow programmed rest before, and provide average of 2-5 readings |
Decreases white coat effect
Approximates proper resting BP, with less human error Used in SPRINT, ACCORD, and most other modern RCTs |
Visit takes longer time
Concern that ‘unattended’ BP differs from Office manual resting |
| Home BP | Usually oscillometric monitors, measured by patient at home | Ease of use
Can help uncover white coat or masked effect (but ABPM diagnostic) Promotes self-awareness and empowerment |
Accuracy of monitors vary
Patients may not measure BP properly (eg rest) Little guidance on frequency May cause more anxiety, health resource utilization |
| Ambulatory BP Monitoring (ABPM) | BP averaged from oscillometric monitor worn usually over 24 hours (44 hours in dialysis patients) | Considered ‘gold standard’ especially for diagnosis of white coat and masked effect
Can show nocturnal BP pattern |
Logistics/ease
Not universally covered by insurance |
Special Note: Hereafter, “Office BP” refers to “Office Manual Resting”
A lot of attention is focused on BP measurement. Office measurement is divided into conventional and unattended Office BP measurement. They do not seem enamored with unattended BP measurement, and state that conventional Office SBP readings might be 5 – 15 mm Hg higher compared to unattended measurement (which was frequently done in SPRINT, and covered in a previous NephMadness). The reference cited is actually comparing manual to the BPTru device, not the Omron HEM 907XL, which was the one used in SPRINT. One might argue, needless to say, that if the Omron HEM 907XL was used, and its usage helped in correct assessment and lowering CV events, then that is what really matters. More recent research also suggests that the difference between automated oscillometric and manual measurements is not a matter of mere subtraction, but rather that it is a wide scatter, implying that the method used in SPRINT should be used if you are applying those results to a wider population. Since these guidelines do use some evidence from SPRINT, the disavowal of the SPRINT-like measurement of BP and using arithmetical manipulation for conversion of the data from SPRINT are a bit incongruent.
In the figure below, the first column shows how the difference between routine and research grade BP measurement is widely scattered, and a simple 5 – 15 mm Hg subtraction is unwise:

Bland–Altman plot showing the mean differences between various blood pressure (BP) recordings and their limits of agreement. Figure 1 from Agarwal et al, JAHA
On Home BP measurement, the EU Guidelines are far more embracing and expansive. Home BP mean is assumed to correspond to the daytime (or awake) mean on ambulatory BP monitoring (ABPM, also covered in NephMadness 2016) and the threshold of > 140 mm Hg for definition of hypertension corresponds to > 135 mm Hg for Home BP or daytime ABPM, and to > 120 mm Hg for night-time (or asleep) ABPM, and corresponds to > 130 mm Hg for 24-hour ABPM overall.
Unlike the US Guideline table 11, the EU Guidelines do not provide non-evidence-based ‘corresponding’ numbers for different levels of BP. For Home BP in particular, the guidelines suggest using a semi-automatic, validated monitor, preferably for 6-7 consecutive days with 2 measurements taken each time. After an excellent discussion of the advantages of ABPM, the EU Guidelines actually suggest clinical indications for Home BP monitoring or ABPM, including:
- Suspicion for white coat hypertension (eg grade I hypertension in office or markedly high Office BP with no end organ damage)
- Suspicion of masked hypertension (high normal Office BP or normal BP with end organ damage)
- Postural or postprandial hypotension
- Evaluation of resistant hypertension, or just for proper evaluation of BP control, especially in patients with high cardiovascular risk
- Investigation of exaggerated BP response to exercise
- Further evaluation of individuals with variability in Office BP
- Assessment of nocturnal dipping (ABPM, not Home BP)
These indications are useful for the practicing clinicians to consider, and suggest that there needs to be a wider role for ABPM use in general.
What is Hypertension?
Do recent data from an American trial that was stopped early change decades of research and literature about blood pressure? Can there be a single goal for BP control, whether for a 30-year old-person with no other comorbid conditions, or for a 65-year-old with a history of a stroke and diabetes, or for a frail 85-year-old living in a nursing home? Of course their risk of having a cardiovascular disease event varies, and their risk of having an adverse events varies – and so that should guide our therapeutic plan. One cannot and should not take a simplistic and naive ‘one size fits all’ approach to a complex condition like hypertension.
Hypertension in the EU Guidelines is defined as a BP > 140/90 mm Hg (unless the patient is aged > 80 years, when hypertension is redefined as > 160/90 mm Hg); how we treat hypertension and to what target BP depends on the individual patient and their cardiovascular risk. Let’s see how that works out in chronic kidney disease (CKD).
Management of Hypertension in CKD
Section 8.12 in the EU Guidelines deals with hypertension and CKD. The bottom line?
- A IA recommendation to treat BP > 140/90 mm Hg with lifestyle and medications, targeting SBP of 130 – 139 mm Hg.
- A IA recommendation again to consider renin-angiotensin system (RAS) blockers as being more effective in reducing albuminuria and hence to be used preferentially in patients with CKD with moderately or severely elevated microalbuminuria.
- Another IA recommendation to use the combination of a RAS blocker and either a diuretic or a calcium channel blocker (CCB) as initial therapy.
To balance the IA recommendations, there is a IIIA recommendation to avoid combining two RAS blockers (one of the teams in ‘Missteps in Nephrology,’ NephMadness 2016). Lastly, despite the 130-139 mm Hg target aim for systolic BP, there is a soft IIa Consensus recommendation for individualizing treatment while paying attention to kidney function and electrolytes. The guideline does not specify whether that refers to targeting SBP below 130 mm Hg if tolerated or to staying higher than 140 mm Hg if BP lowering is poorly tolerated.
The evidence base used to make these recommendations is quite interesting – and mostly dates to 2011 or before. They quote a ‘recent’ meta-analysis, from 2013, which reported lower incidence of end-stage kidney disease (ESKD) with intensive BP lowering in those with albuminuria, as well as another ‘more recent’ (2017) meta-analysis, which reported lower all-cause mortality in patients with CKD with intensive BP lowering. However, they use a 2011 meta-analysis (predating SPRINT) which failed to demonstrate that a BP target of < 130/80 mm Hg improved clinical outcomes compared to a target of < 140/90 mm Hg for their main recommendation. None of the SPRINT-CKD data that were presented briefly in the main manuscript and were separately published in Sep 2017 are mentioned. Reflecting minimal data, there is no discussion of management of hypertension in hemodialysis or transplant.
Other Highlights of the EU Guidelines
There is a lot of useful advice packed into the rest of the guidelines. Some handpicked tidbits include:
- For lifestyle changes, the EU Guidelines also suggest assessing for and monitoring binge drinking, and recommend smoking cessation for cardiovascular protection.
- Resistant hypertension is classified as BP > 140/90 mm Hg assessed using Home BP measurement, despite three or more drugs including a diuretic at optimal doses, and after assessment of adherence.
- Table 33 (and the text in section 10.4) has very useful suggestions for managing non-adherence. Some of the key aspects: work with nurses and pharmacists, check accessibility, simplify medication regimen, and spend more time with the patient.
- For the elderly, which seems to be defined as aged > 65 years, the target BP is 130 – 139/70 – 79 mm Hg – but not less than 120/70 mm Hg and only if tolerated.
- Throughout the management recommendations, pay attention to two features quite unique to the EU Guidelines (as compared to the US guidelines). First, they almost always present a a floor blood pressure, ie do not go below a systolic BP of 120 mm Hg. Second, different targets are presented for systolic and for diastolic BP throughout all the sections.
US Guideline

Copyright: KateChe / Shutterstock
Workgroup
The US Guideline refer to the first foray of the American College of Cardiology (ACC) and the American Heart Association (AHA), accompanied by the AAPA, ABC, ACPM, AGS, APhA, ASH, ASPC, NMA, and PCNA, into the murky and treacherous waters of hypertension guidelines. The Joint National Committee (JNC) last produced guidelines in 2003 (the seventh report), with the National Institutes of Health halting financial support while the eighth report was being developed. The publication in 2013 that is referred to as JNC-8 was not really a JNC-8, but rather a report from the panel members of the JNC-8 (or JNC wait or JNC late as it was sometimes referred to), something which makes sense once you also read the disavowal from the minority report (written by many SPRINT investigators). SPRINT, in more ways than one, represents a schism, a break from the past, and a divide among hypertension specialists and guidelines.
Like the EU Guidelines, the US Guideline taskforce does not have official input from the American Society of Nephrology, or other kidney professional societies (seriously, among an alphabet soup of societies more complicated than NCAA basketball conferences, there is no input from a nephrology society), although it did include one nephrologist as well as two patient/lay representatives on the writing committee.
Evidence Review
The ACC/AHA did conduct its own evidence review, which was simultaneously published with the guideline, and they reviewed related guidelines from other organizations. All this, plus a lot of very helpful information including links to their app, slidesets, summaries etc, are available freely on their guideline hub website.
Guideline Organization
For rating the evidence and the recommendation, the US Guideline uses a Class of Recommendation (COR, ranging from I to III) and a Level of Evidence (LoE, ranging from A to C, with a R [randomized trials], NR [non randomized studies], LD [limitations in design], or EO [expert opinion] as additional monikers). So the recommendations could be:
- IA: treatment is recommended based on high quality evidence from more than 1 trial
- IIB (COR) B-NR (LoE): for something being reasonable to do, based on mechanistic studies, or observational studies or registry data with limitations, or IIB C-EO: something being reasonable based on expert opinion
- IIIA: evidence that something is not recommended or even if something is harmful
- on the basis of more than one trial
Home BP Measurement
Sections 4.2 to 4.4 deal with BP measurement, and start off with a IA level recommendation to use Home BP measurements to confirm diagnosis of hypertension and to guide titration of BP-lowering medications. For the latter, the recommendation stems from the excellent systematic review which suggested Home BP monitoring improves BP control, potentially by overcoming therapeutic inertia.
There is a fascinating table (Table 11) which provides corresponding values for Home, Office, and ABPM (day, night, and overall), although there is no discussion about the data supporting this table. It is interesting because this table suggests that the difference between Office and Home BP varies based on the baseline Office BP. For example:
- At normal BP (eg Office BP 120/80 mm Hg) Home BP is usually 120/80 mm Hg and so is daytime (awake) ABPM, but
- At Office BP of 140/90 mm Hg, Home BP and daytime ABPM are 135/85 mm Hg, but overall 24 hour ABPM is 130/80 mm Hg with a night-time (asleep) BP of 120/70 mm Hg
- The difference between Office and Home BP is even higher at Office BP of 160/90 mm Hg; at this level, the corresponding Home BP would be 145/90 mm Hg as would overall and daytime ABPM, while nighttime BP is 140/85 mm Hg (which mathematically doesn’t make sense as the overall average includes day and night)
The supporting citations are those that show which thresholds of ABPM are associated with outcomes rather than ‘corresponding values’ as stated in the table legend. Perhaps what this table intended to show is what should be considered a diagnostic threshold for Home BP or ABPM corresponding to a certain Office BP threshold, but even then the nighttime values hardly make sense; given the AHA/ACC threshold of 130/80 mm Hg to define hypertension, perhaps the information presented could have been restricted to that single row/level?

Visual Abstract by @jiahweing on Kikuya et al
Unlike the EU Guidelines, the US Guideline does not provide indications for performing ABPM for reasons other than to diagnose hypertension and achieve long-term control, although they do discuss that ABPM can help for assessment of nocturnal hypertension and variability. This is potentially understandable given that the Center for Medicare and Medicaid Services currently only pays for ABPM for the diagnosis of white coat hypertension.
What is Hypertension?
As John Maynard Keynes is oft-claimed to have said, ‘When the facts change, I change my mind.’ Science is not static, burnt on stone tablets and never changing. As more knowledge becomes available, we should constantly examine our priors and not remain anchored to our beliefs. Despite all the deniers out there, we should acknowledge that SPRINT was the landmark hypertensive trial of this decade, and will remain in the Hypertension Hall of Fame forever. The investigators chose an innovative – and easy-to-apply to real world – method of measuring BP, chose patients who would be at high cardiovascular risk, and made sure there was a clear separation of BP between arms. Patients at very high risk of adverse events were carefully excluded, such as those who were institutionalized, those with standing BP < 110 mm Hg (how often does one measure that?), or someone unable to stand (ie in a wheelchair), anyone with a recent (within 3 month) cardiovascular event or procedure, and those with an expected life expectancy < 3 years. That these strengths are held up as flaws tells you more about the SPRINT-deniers than about the trial. But the US Guideline does not just toe the sprint line of 120 mm Hg; they do take a more nuanced look at the hypertension threshold and proffer a target BP of < 130/80 mm Hg instead. Read the fine print – and appreciate that a simple, across-the-board 130/80 mm Hg makes it so much easier for physicians and patients alike.
Management of Hypertension in Chronic Kidney Disease
Section 9.3 of the US Guideline covers management of hypertension in people with CKD. First, in a major difference from the EU Guidelines, the US Guideline recommends a goal of < 130/80 mm Hg irrespective of albuminuria (Class of Recommendation I, Level of Evidence B-R for systolic, C-E for diastolic – refer to Guideline Organization section above). Secondly, in patients with CKD (either GFR < 60 mL/min/1.73 m2 or with urine albumin to creatinine ratio > 300 mg/g or the equivalent), they recommend treatment with an ACE inhibitor first, to slow kidney progression (IIA recommendation). Lastly, treatment with an ARB is a IIb recommendation in the same patients, as being reasonable if the ACE inhibitor is not tolerated (IIB C-EO).
Why should the goal BP be < 130/80 mm Hg and not < 140/90 mm Hg, as the Europeans suggest? This relies on the systematic review conducted by the US Guideline Taskforce, as well as data from SPRINT, in which the subgroup with eGFR <60 mL/min/1.73 m2 at baseline (28% of SPRINT overall) seemed to have the same benefit with intensive blood pressure control as the overall population. Subsequent to these guidelines, we have had the publication of the detailed cardiovascular outcomes in the CKD population which confirm and expand on those results. They do give a passing nod to the same evidence that existed prior to SPRINT (which the EU Guidelines rely on) but take the position that SPRINT supersedes these. Not much is offered on why ACE inhibitors precede ARBs (which should be used in ACE-intolerant patients). This may be a missed opportunity for citing this network meta-analysis in AJKD, which suggests possible superiority of ACE inhibitors to ARBs in CKD. A contrary take was made in a recent review, favoring ARBs on the basis of similar efficacy and fewer adverse effects.
More importantly, while there is no section on management of hypertension in hemodialysis in the US Guideline, there is a section (9.3.1) on management of hypertension after renal (yes they call it renal, not kidney) transplantation (maybe evidence that more nephrologist input in the guideline was needed?). Two quick recommendations (both IIA)
- Goal BP should still be less than 130/80 mm Hg, the same as other CKD populations.
- A IIa recommendation suggesting that it is reasonable to use calcium channel blockers (CCB) on the basis of better kidney survival.
A Cochrane review is cited to favor CCBs, and, not cited, but relevant here, is a recent systematic review in AJKD to suggest RAS blockade is nothing special in this population. For the goal of 130/80 mm Hg itself, the group acknowledges the lack of any trials addressing goal BP, but finds no reason from diverging the BP goal from other patients with CKD, given that all patients with a functioning kidney transplant have a solitary kidney by definition have CKD.
Other Highlights of the US Guideline
- On lifestyle, the US Guideline does not mention smoking or binge drinking (they do advise keeping alcohol intake down for BP reduction, as do the EU Guidelines), but do mention enhanced potassium intake, and discuss benefits of dynamic and isometric resistance exercise in addition to aerobic exercise (that the EU Guidelines restrict to).
- Resistant hypertension is now classified as BP > 130/80 mm Hg despite three or more drugs, including a diuretic at optimal doses.
- In the non-institutionalized elderly, the target BP is < 130/80 mm Hg (no floor) as a IA recommendation, and for those with high comorbidity burden or limited life expectancy, a team-based approach for individualized assessment is recommended (IIA, EO).
Primary Aldosteronism
Though Thomas Addison described the effects of adrenal insufficiency in 1855, it took almost a century for the converse to be described, that of aldosterone excess, by Jerome Conn in 1954. (1954 incidentally is also when Frank Selvy scored 100 points in an NCAA game, and was drafted by the Baltimore Bullets, who ignominiously folded soon thereafter). The actual description of mineralocorticoid activity arising from the adrenals predates Conn by a couple of years, from Grundy, Simpson, and Tait. Nevertheless, all this happened half a century ago, so why should NephMadness be interested in the topic now? From diagnosis to management, there has been a renaissance in primary aldosteronism. But what matters more: diagnosis or management? Let’s jump in.
Hyperaldo Diagnosis
Primary aldosteronism is a cause of secondary hypertension. How common is it? Actually not that common, but in today’s world with high sodium consumption, it doesn’t take much of an increase in aldosterone to be a causative factor in increased blood pressure. There are empiric data to support this: pre-1998 prevalence of primary aldosteronism is sub-10%, but post-1998 is in the teens. The prevalence increases as the severity of hypertension increases. These data are from case series, however, and may reflect detection and awareness bias. Indeed, some misguided folks may jump the gun and say, why bother with detecting and diagnosing primary aldosteronism, when empiric mineralocorticoid antagonists (MRA, think spironolactone or eplerenone) can be used instead? But is blood pressure (BP) control all that matters – should we not keep our eyes on the ball, the reason why we treat high BP at all, that is a reduction in cardiovascular (CV) events? (And no, despite the #BlueRibbonFail of NephMadness 2016, the reason we treat high blood pressure is for CV outcomes, not the kidneys). Let us try to understand who might have primary hyperaldosteronism, and the nuances of how to properly diagnose this condition to understand why all this matters.

Copyright: Shidlovski / Shutterstock
Screening and Diagnosis
There are many screening guidelines, focusing mainly on patients with difficult to control hypertension with some signs that should make one suspicious. Some common screening criteria include:
- Resistant hypertension (defined by the US Guideline as BP > 130/80 mm Hg with 3 or more drugs at optimal dosages, including a diuretic)
- Spontaneous or diuretic induced hypokalemia
- Hypertension with an incidentally noted adrenal tumor
- Family history of primary aldosteronism
- Family history of premature stroke (age < 40 years)
- Hypertension and sleep apnea
What are the next steps? Though the aldosterone-to-renin ratio is often mentioned as a good tool, it’s wise to look at it one step at a time. Review the renin first (and make sure you say it right, REE-nin, not RENN-in, even in your head) and then go on to aldosterone. See Figure 2 from this useful review for a step-by-step guide.
Secondly, like any test, renin-aldosterone testing has false positives and false negatives. Chief among the causes of false negative tests are
- the presence of hypokalemia (which will suppress aldosterone production and is why it is important to fix potassium and recheck if high suspicion)
- Concurrent use of MRAs (and less often with angiotensin converting enzyme inhibitors and angiotensin II receptor blockers)
- Concurrent use of beta-blockers, diuretics, or with sodium restriction
That last point also plays into the role of the salt loading test. Sodium loading and volume expansion should suppress aldosterone under physiologic conditions, but not when aldosterone production is autonomous. This is recommended for those with ‘probable’ primary aldosteronism; those with the trifecta of spontaneous hypokalemia, suppressed renin, and elevated plasma aldosterone do not need additional confirmatory testing. The utility of the sodium loading test is also underscored in this discussion – it’s all about inappropriately elevated aldosterone levels.
PRA or DRC?
A quick note about units, assays, and ratios. The mainstay of measurement of renin used to be plasma renin activity (PRA) and this is being rapidly replaced by renin mass, measured as direct renin concentration (DRC). PRA is not actually a measurement of renin levels; as its name implies, it relies on conversion of angiotensinogen to angiotensin I. Since this is a rate, it is expressed in units not of concentration, but of speed (ng/ml/hour or pmol/minute). Some argue that PRA therefore depends on the amount of the substrate which may not be a constant. Another confounder could be the use of a renin inhibitor (aliskiren), which will inhibit the PRA assay and result in a low activity measurement, even if DRC is markedly elevated.
Standardization of laboratory methods in the face of so many moving parts is a bit tricky, to say the least. Hence the rise of the DRC (also sometimes referred to as plasma renin concentration [PRC] or direct renin mass). DRC, as compared to PRA, is more reproducible within and between labs, since it is faster and methodologically simpler. But what happens to the beloved aldosterone-to-renin ratio – an addition of a layer of complexity to the possible permutations and combinations of units? A quick and handy cheat sheet follows below:
| PRA (ng/ml/hour) | PRA (pmol/L/minute) | DRC (mU/L) | DRC (ng/L or pg/mL) | ||
| Plasma Aldosterone Concentration (ng/dL) | Low threshold | 20 | 1.6 | 2.4 | 3.8 |
| High threshold | 40 | 3.1 | 4.9 | 7.7 | |
| Plasma Aldosterone Concentration (pmol/L) | Low threshold | 750 | 60 | 91 | 144 |
| High threshold | 1000 | 80 | 122 | 192 |
Table: Suggested thresholds for the ARR (aldosterone-renin ratio). Use of the low threshold will result in more false positives, a high threshold more false negatives.
Localization
Localization is important when one is looking for a cure – or if the blood pressure is so bad that surgery leading to an improvement in BP would be desirable. Localization is especially important as surgery will not be as helpful for bilateral adrenal hyperplasia. Obvious, but important. An aldosterone producing adenoma (APA) is reported to be present in about 20% to 50% of all cases of primary aldosteronism. This somewhat wide range stems from what definition of primary aldosteronism is used, apart from the methods used to localize. Physiology trumps anatomy for localization, and adrenal venous sampling is considered more useful than imaging. It is easier said than done though; after all, catheterizing adrenal veins and labeling all the samples correctly is not as easy as doing a CT scan. Adrenal venous sampling also carries a certain non-negligible complication rate, such as adrenal hemorrhage, which can occur even in experienced hands, though overall long-term outcomes do seem benign. Lastly, AVS needs to be conducted in specialized centers, as many things need to be carefully orchestrated, and that may put it out of reach of many patients who need it. It is also inconclusive sometimes.
With the advances in imaging, surely imaging would be able to pinpoint functional adenomas that can be excised? The Subtyping Primary Aldosteronism: A Randomized Trial Comparing Adrenal Vein Sampling and Computed Tomography Scan (SPARTACUS) did try to do this, and did not show a difference between imaging versus adrenal venous sampling in terms of BP control at one year. But several caveats should give us pause on this otherwise well-done trial. The outcome was BP control with a defined dose of drugs, not biochemical control. Indeed, patients with APA can have good BP control with medical treatment, and some patients with BAH can have improvement in their BP with unilateral adrenalectomy. Hence, BP control is a somewhat noisy outcome, though one could argue that maybe that is really all that matters. But no, we are nephrologists, and we need precision, backed by numbers. So adrenal venous sampling it is for proper localization. (Editor’s note: for more substantive critiques of SPARTACUS, see these commentaries). A useful guide for how to perform adrenal venous sampling is provided in this review.
Genetics
Could genetics help us nail a three-pointer and avoid the localization uncertainty? Next Generation Sequencing was in NephMadness 2015 for a reason. There truly is a lot we know about the genetics of primary aldosteronism, reflecting progress over the last decade. APAs express aldosterone synthase (CYP11B2) and often harbor somatic mutations, and now we have been able to identify some of them. Mutations in the KCNJ5 (Potassium channel, inwardly rectifying, Subfamily J, Member 5) can result in increased sodium conductance and cell depolarization causing calcium entry, signaling cell proliferation and aldosterone production. The same group a few years later described a gain-of-function mutation in the voltage dependent L-type, alpha 1D subunit calcium channels (CACNA1D) which is a sufficient stimulus for aldosterone production and cell proliferation in adrenal glomerulosa. Other mutations in ATP1A1, and ATP2B3 have been described, which together with KCNJ5 and CACNA1D, represent the majority of APAs. But primary aldosteronism can also run in families, thus not just from somatic, but germline mutations. Some of them manifest as bilateral adrenal hyperplasia and some as APAs. See the table below for the subtypes (which will probably expand in the coming years).
| Characteristics | |
| Familial Hyperaldosteronism – I | CYP11B1-B2 hybrid
Glucocorticoid-Remediable Aldosteronism |
| Familial Hyperaldosteronism – II | CLCN2 mutation, can manifest as APA or BAH |
| Familial Hyperaldosteronism – III | KCNJ5 mutations, results in BAH and early onset primary aldosteronism with severe hypokalemia |
| Familial Hyperaldosteronism – IV | CACNA1H mutation, early onset primary aldosteronism |
| primary aldosteronism associated with seizures and neurological abnormalities (PASNA) | CACNA1D mutations, described de novo, associated with seizures and neurologic abnormalities |
Table 2: Types of familial hyperaldosteronism. Almost all are autosomal dominant, though with varying penetrance.
How about the vast majority of BAH patients, who don’t have a family history (‘idiopathic’ hyperaldosteronism)? Curiously, they often have aldosterone-producing cell clusters (APCC) rather than a diffuse non-nodular hyperplasia. More interestingly, these APCCs have been reported to have mutations in CACNA1D, and less commonly KCNJ5. Could they represent future APAs? What stimulus drives these somatic mutations? The more we know, the more we know what we don’t know.

Visual Abstract by @ericau on Choi et al
Conclusion: The Opposition
This is a non-starter. How can one even discuss the management of primary aldosteronism without properly diagnosing it? And the diagnosis makes the treatment easy-peasy. Lateralized? Take out the adrenal. Otherwise give MRA. What else is there in managing primary aldosteronism?
Hyperaldo Treatment
Dichotomizing management into medical and surgical is such a Manichean reasoning. There are many shades of grey that lie in between, as well as with proper medical management, that such simplistic thinking does not even scratch the surface.

Copyright: Shidlovski / Shutterstock
Surgical Management
Got an APA? Take the offending gland out. Laparoscopic technique works well. But wait, is surgery all that superior, even in the setting of a unilateral aldosterone-producing adenoma (APA), if one can get BP down and potassium up to desirable levels with medical management? Maybe there is an advantage, and not just in aldosterone levels, but in clinically relevant outcomes. In a cohort including 400 patients treated with MRA and 120 who underwent surgical adrenalectomy, the rate of decline of kidney function was higher in those treated medically, as well as development and worsening of albuminuria in those who had primary aldosteronism and diabetes treated medically. Secondly, in another analysis from the same group, new onset atrial fibrillation was lower in those who had surgery compared to those treated medically.
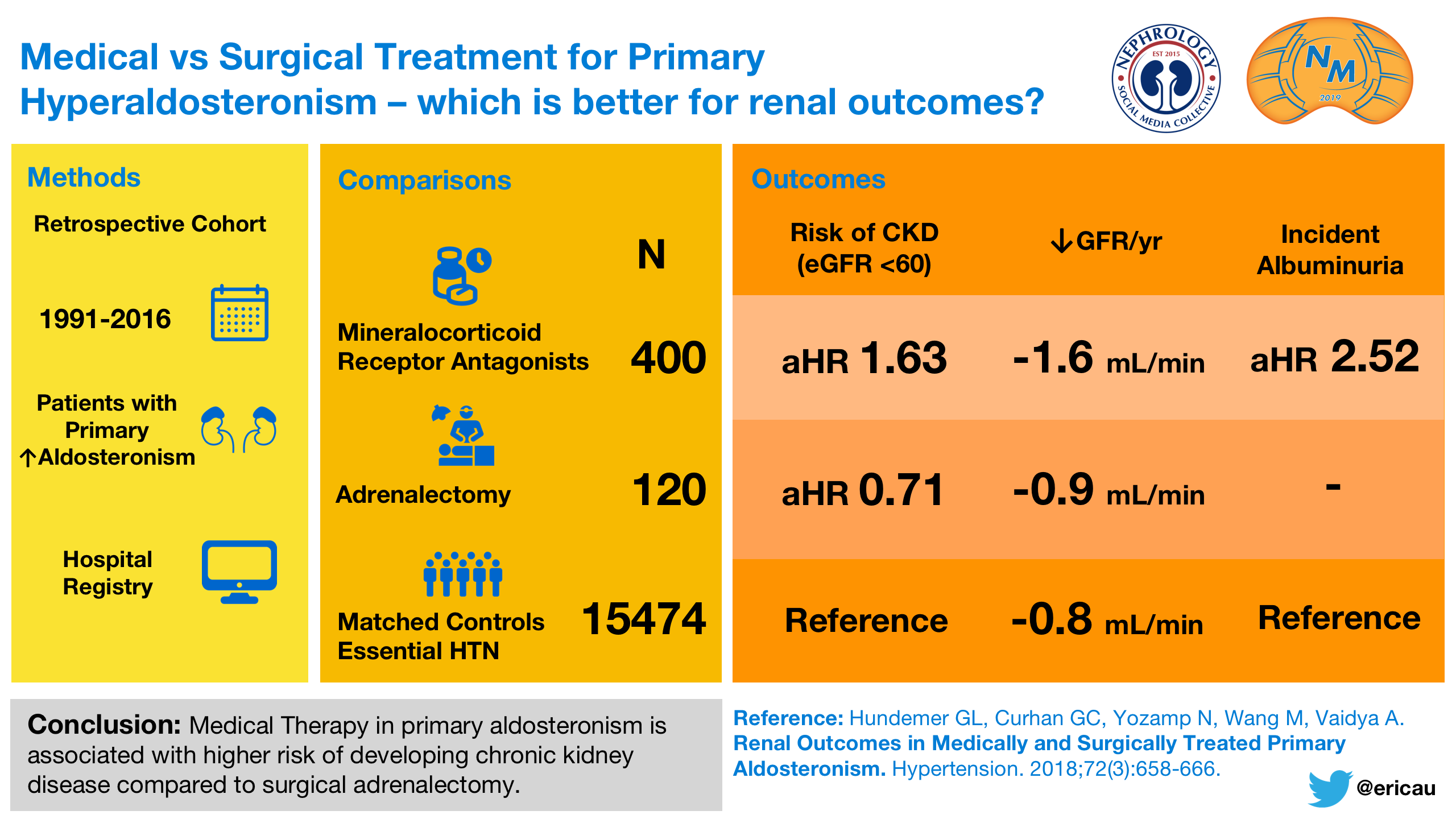
Visual Abstract by @ericau on Hundemer et al
Empiric Spironolactone Therapy
Mineralocorticoid antagonist (MRA) therapy is the mainstay in patients with primary aldosteronism. But primary aldosteronism represents only about 10% of patients with resistant hypertension. And spironolactone works for all of them, so why even bother with diagnosis? In the PATHWAY-2 trial, among patients with hypertension uncontrolled on the standard A-C-D combination, spironolactone produced the largest BP decrease compared to bisoprolol and doxazosin. Moreover, spironolactone was more potent at BP lowering than the other two throughout the spectrum of baseline renin levels (except a small subset with very high renin levels), so why bother diagnosing primary aldosteronism and measuring renin (and converting ng/minute into pmol/parsec or whatever)?

The vertical dashed line shows that the blood pressure fall on bisoprolol numerically exceeds that on spironolactone only in the top 3% of the renin distribution. Figure 3 from Williams et al, the Lancet.
Subclinical or Non-Classical Primary Aldosteronism
This point becomes even more painfully obvious once one realizes that the dichotomizing ‘primary aldosteronism’ and ‘not primary aldosteronism’ is far from perfect. A spectrum describes the role of aldosterone more accurately. This was most clearly demonstrated in a recent report from the Hypertension Pathotype (HyperPATH) cohort. Biochemically overt primary aldosteronism was found in individuals who are normotensive, and in those with mild to moderate hypertension, suggesting a process of subclinical aldosteronism in these individuals. Indeed, aldosterone-producing cell clusters (APCCs) are reported to be found in autopsy studies of individuals without hypertension, providing an anatomic correlate to this biochemical observation. An epidemiological tie-in is the observation of elevated cardiovascular risk in these patients – higher than those with normotension and no evidence of biochemical primary aldosteronism. Similar findings about the prevalence of biochemically overt primary aldosteronism being more than zero (but in the 3-5% range) emerge from patients with mild-moderate hypertension in other cohort studies. Thus, the strict screening criteria you read above may not be good enough. Moreover, its begs the question: should MRA make it to the Big Dance as a first line of therapy for hypertension, hitherto restricted to A-C-D? The absence of high quality randomized trials gives us pause, but it remains an excellent question to ponder.
“So you have established primary aldosteronism, is there more to just writing a prescription for an MRA?”
Of course there is. Not everyone tolerates spironolactone, for one. It can cause spotting in menstruating women and breast tenderness at high doses. Moreover, it should be combined with contraception in women of childbearing age given the possibility of teratogenicity to male fetuses. In men, it can cause gynecomastia and sexual dysfunction. As in women, these adverse effects are dose-dependent, and may not manifest immediately.
Why not give eplerenone to everyone instead? It’s not covered by many insurance plans and costs far more than the few pennies or cents that spironolactone does. Eplerenone is less potent, so one requires double the dose, split into a twice a day schedule. As a recent review wisely puts it: “The most common mistakes made in prescribing spironolactone for primary aldosteronism are starting at too high a dose and titrating up too quickly. The most common mistake in prescribing eplerenone for primary aldosteronism is not using a high enough dose and not dividing the dose twice daily.” Doses used in primary aldosteronism for MRA may need to go up to 200 or even 400 mg.
MRA Alternatives
If MRAs work, why not use a downstream inhibitor of the channel where aldosterone actually acts, renal outer medullary potassium channel (ROMK)? Amiloride and triamterene do block the ROMK channel; could they be sufficient if someone cannot tolerate MRA? In a substudy of PATHWAY-2, 146 patients received amiloride as a run-out after completion of the main trial (which reported the superiority of spironolactone over bisoprolol and doxazosin in BP reduction). 10 mg of amiloride resulted in a similar reduction of BP (~ 20 mmHg from baseline) as was seen with 25 mg spironolactone in these patients. Needless to say, these patients were those with resistant hypertension and not primary aldosteronism and we do not have data on clinical outcomes, but these are useful data to consider if one starts running out of options.
Targeted Therapy
The dose titration mentioned in the previous section refers to MRA as being used for the clinical phenotype of hypertension and hypokalemia. However, there is an underlying biochemical phenotype: as exemplified by the elevated and renin-independent production of aldosterone. This indeed causes the suppression of renin (hence the use of aldosterone-renin ratio for diagnosis of primary aldosteronism), and could potentially be used to guide therapy. How does one know if the aldosterone effect has been adequately blocked with the dose of an MRA? By checking if the renin is now ‘un-suppressed’! This physiological insight was tested in a longitudinal cohort of 600 patients. As expected, the incidence of cardiovascular events was almost twice as high in patients with primary aldosteronism compared to those with essential hypertension. However, on grouping the patients with primary aldosteronism into those whose renin remained suppressed and those whose renin was unsuppressed, the increase in cardiovascular events was only seen in the former group. Similarly, the development of new onset atrial fibrillation was also higher with suppressed renin. Though these were retrospective studies and not trials, this strategy of following renin seems quite coherent to use while titrating MRA in patients with primary aldosteronism.
Conclusion
As can be seen, there is so much more to management of primary aldosteronism than surgery and MRA. Surgery seems to have advantages over purely medical therapy in cases of APA. There many nuances about MRA usage that we have now gleaned and will continue to learn over the coming years. Yes, one needs to know the diagnosis, but if you don’t understand the nitty-gritty of the management pearls highlighted above, you might as well not have truly diagnosed primary aldosteronism. Period.
– Executive Team Member for this region: Matthew Sparks, AJKD Social Media Advisory Board member. Follow him @Nephro_Sparks
How to Claim CME and MOC
US-based physicians can earn 1.0 CME credit and 1.0 MOC point for reading this region.
- Register/log in to the NKF’s Professional Education Resource Center (PERC).
- Review the activity and accreditation information.
- Click “Continue” and review Course Instructions.
- Complete Post-Test. Please note: By selecting “Yes” to the participation questions for each region, the corresponding Post-Test questions will appear. Click “Save Draft” to save your responses and finish later. When you are ready to submit your answers, click “Preview” to review all responses, then click “Submit.”
- Click “Next” to complete the Evaluation form, then click“Submit.”
- Claim 1.0 CME credit and 1.0 MOC point per region (up to 8.0 total for 8 regions of NephMadness).
- Print your certificate.
- Review the Post-Test answers and rationale.
The CME and MOC activity will expire on June 15th, 2019.
Submit your picks! | #NephMadness | @NephMadness | #HTNRegion

Amazing information! Keep sharing such useful and beneficial blogs about hypertension management. Thank you!