#NephMadness 2024: Metabolic Acidosis Region
Submit your picks! | NephMadness 2024 | #NephMadness
Selection Committee Member: Nimrit Goraya
Nimrit Goraya is a Program Director of Nephrology Fellowship at Baylor Scott and White in Temple, TX and an Assistant Professor of Medicine (Nephrology) at Baylor College of Medicine. Her research is focused on the dietary benefits of plant-based diet and metabolic acidosis in CKD and her mission is to continue to improve care, awareness, and outcomes for her patients.
Writer: Taha Mohamed Djirdeh
Taha Mohamed Djirdeh is a second-year fellow at Washington University in St. Louis. His areas of clinical interest include peritoneal dialysis and the overlap of nephrology and cardiovascular issues.
Writer: Marco Thierry @MarcoThierry1
Marco Thierry is a second-year nephrology fellow at Washington University in St. Louis, where he currently serves as chief fellow. He will be going to Billings Clinic in Montana to serve as a community nephrologist . His areas of interest include ESKD and home modalities.
Competitors for the Metabolic Acidosis Region
Team 1: Metabolic Acidosis in CKD/Outpatient
versus
Team 2: Metabolic Acidosis in AKI/ICU

Image generated by Evan Zeitler using Image Creator from Microsoft Designer, accessed via https://www.bing.com/images/create, January, 2024. After using the tool to generate the image, Zeitler and the NephMadness Executive Team reviewed and take full responsibility for the final graphic image.
Nerding out about acid-base physiology is an endeavor most nephrologists are proud of. I mean, who else other than salt whisperers and urine enthusiasts would take Burton Rose’s seminal textbook and create a book-club podcast on each chapter? Whether it’s an overly detailed explanation of the Henderson-Hasselbach equation or a multifaceted discussion about bicarbonate handling in the nephron, disorders of pH are a big reason we love the game.
Speaking of acid-base disorders, none are more prevalent or as misunderstood as our top prospect this year: metabolic acidosis! In this region, we look at metabolic acidosis in two very different kidney settings and populations to determine which emerges the victor!
Team 1: Metabolic acidosis in CKD/outpatient

Copyright: La_Mar/Shutterstock
One may initially believe that managing metabolic acidosis in the outpatient setting is a pretty dry topic with a paucity of new information. Before we dispute this, a quick primer is needed on the prevalence and mechanism of metabolic acidosis with chronic kidney disease (CKD). If we define metabolic acidosis as a serum bicarbonate as less than 22 mmol/L, the NHANES III study showed that 19% of patients with CKD 4 had this level of acidosis or worse.
Healthy kidneys filter large amounts of bicarbonate each day. Assuming a serum bicarbonate of 24 mmol/L and a GFR of 100 ml/min, the kidney filters more than 3 mol of bicarbonate/day. Reabsorption of this filtered bicarbonate is primarily the role of the proximal tubule. In addition to reabsorbing filtered bicarbonate, we must generate new bicarbonate that is lost in buffering the daily acid load. Regeneration of new bicarbonate through excretion of ammonium or titratable acids occurs further down the nephron in the distal nephron. At the most basic level, metabolic acidosis in advanced CKD is a result of the diminished capacity of these mechanisms.
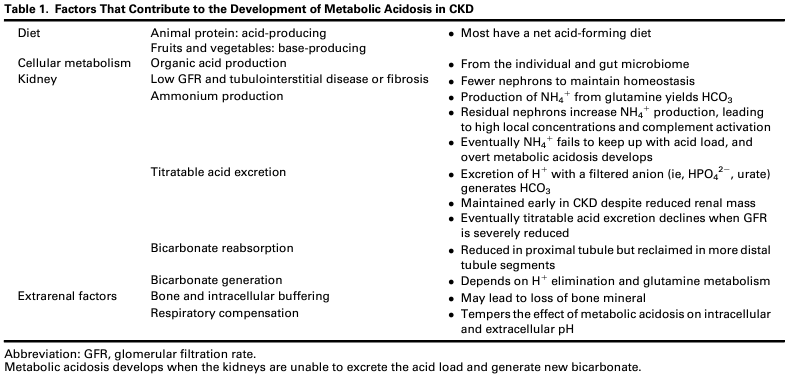
Factors That Contribute to the Development of Metabolic Acidosis in CKD. Table 1 from Ravikumar et al, © National Kidney Foundation.
Why does this matter other than making the bicarbonate number turn red in EPIC? There are several potential consequences of metabolic acidosis that have been observed in clinical studies. Although controversial, these include:
- Adverse bone health
- Decreased muscle mass
- Progression of CKD and GFR decline
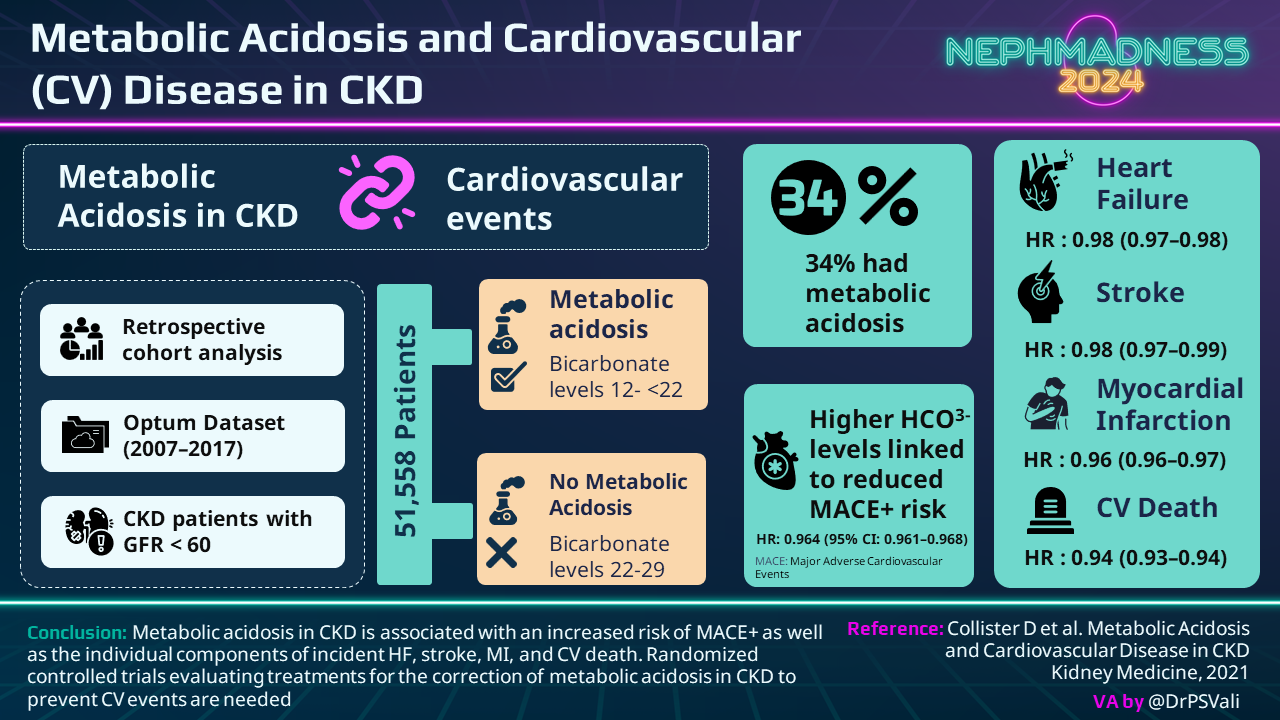
- Higher incidence of major adverse cardiovascular events (MACE) including heart failure, strokes, MI, and death.
Granted, we don’t have large randomized controlled trials for all of these populations (we’ll talk about this more when we get to treatment), but suffice to say that the development of metabolic acidosis in CKD associates with many poor outcomes.
To make things even more complicated, there have been studies attempting to identify patients at risk for these complications even before the serum bicarbonate drops. This “eubicarbonatemic” H+ retention or subclinical metabolic acidosis of CKD has promoted tests like urinary citrate as a marker for acid retention to identify if patients are buffering excess acid prior to bicarbonate depletion. By using urinary citrate levels and estimating H+ retention (based on the difference between observed and expected plasma bicarbonate after an oral alkali load), studies are attempting to identify patients with much earlier levels of CKD 1-2 that may be at risk for the development of metabolic acidosis as their kidney function worsens with time. A small study provocatively suggested the possibility that alkali supplementation resulted in better eGFR preservation over 10 years in eubicarbonatemic patients compared to usual care.
So far, so good! If acidosis is bad, then let’s just give alkali to all of our CKD patients, right? Unfortunately, it’s not that simple. In addition, most bicarbonate trials excluded patients with diabetes, and the data is even murkier with kidney transplant recipients. A Swiss study that evaluated alkali administration for kidney transplant patients with declining graft function showed improvement in correcting metabolic acidosis but no improvement in decline of eGFR at 2 years. Let’s also not forget that oral supplementation of either of these can be a significant pill burden, contribute to polypharmacy, can cause GI side effects like bloating, and can constitute a sodium or potassium load which may not be desirable for patients with advanced CKD. With a typical NaHCO3 supplementation of 1300 mg twice daily in CKD4, we are asking patients to take 4 pills which provide roughly 30 mEq of sodium/day.
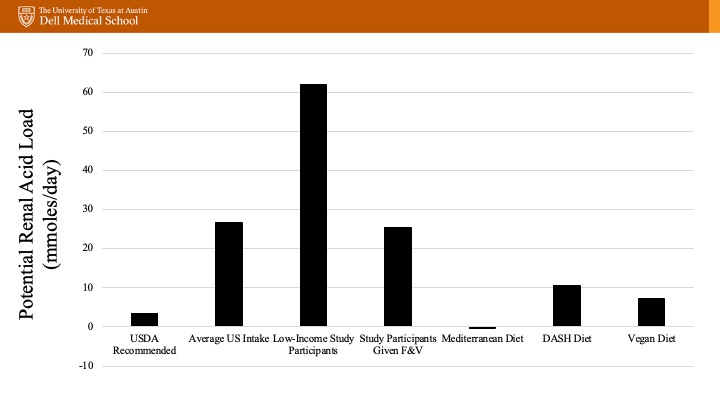
If minimizing medications is a priority, we should remember that development of metabolic acidosis is also dependent on daily acid production which relates to our dietary intake. The potential renal acid load (PRAL) is a way to quantify acid production related to intake. Animal proteins contain amino acids that generate sulfate after oxidation which is a major source of our non-volatile/fixed acid load (i.e. a higher PRAL, or is acid inducing). On the other hand, fruits and vegetables have a significant amount of metabolizable anions including citrate that can consume hydrogen when metabolized. With this in mind, dietary modifications have been studied with promising results. A study in patients with CKD4 and serum bicarbonate < 22 mmol/L randomized to 1 year of sodium bicarbonate therapy at 1 mEq/kg/day vs dietary modification (fruits and vegetables to reduce dietary acid by half) showed interesting results. There was no difference in eGFR, but both groups had improved serum HCO3. The dietary group also showed lower systolic BP, and no evidence of hyperkalemia, suggesting that this could be a useful strategy at least in some patients.
So rather than restricting plant-based diets in all CKD patients due to fear of higher potassium contents, health providers need to promote a healthier diet for patients with CKD where it is safe to do so. Granted, some patients with persistent hyperkalemia will need potassium restriction, but these studies showed that a healthy dietary pattern with higher intake of fruits, vegetables, low-fat dairy foods, whole grains, and fiber coupled with a lower intake of sodium, red meat, and sugar was associated with a 30% reduced odds of kidney damage. The benefits extend beyond acidosis with improvements in BP, phosphorus, glycated hemoglobin, and even proteinuria in patients with healthy dietary patterns!
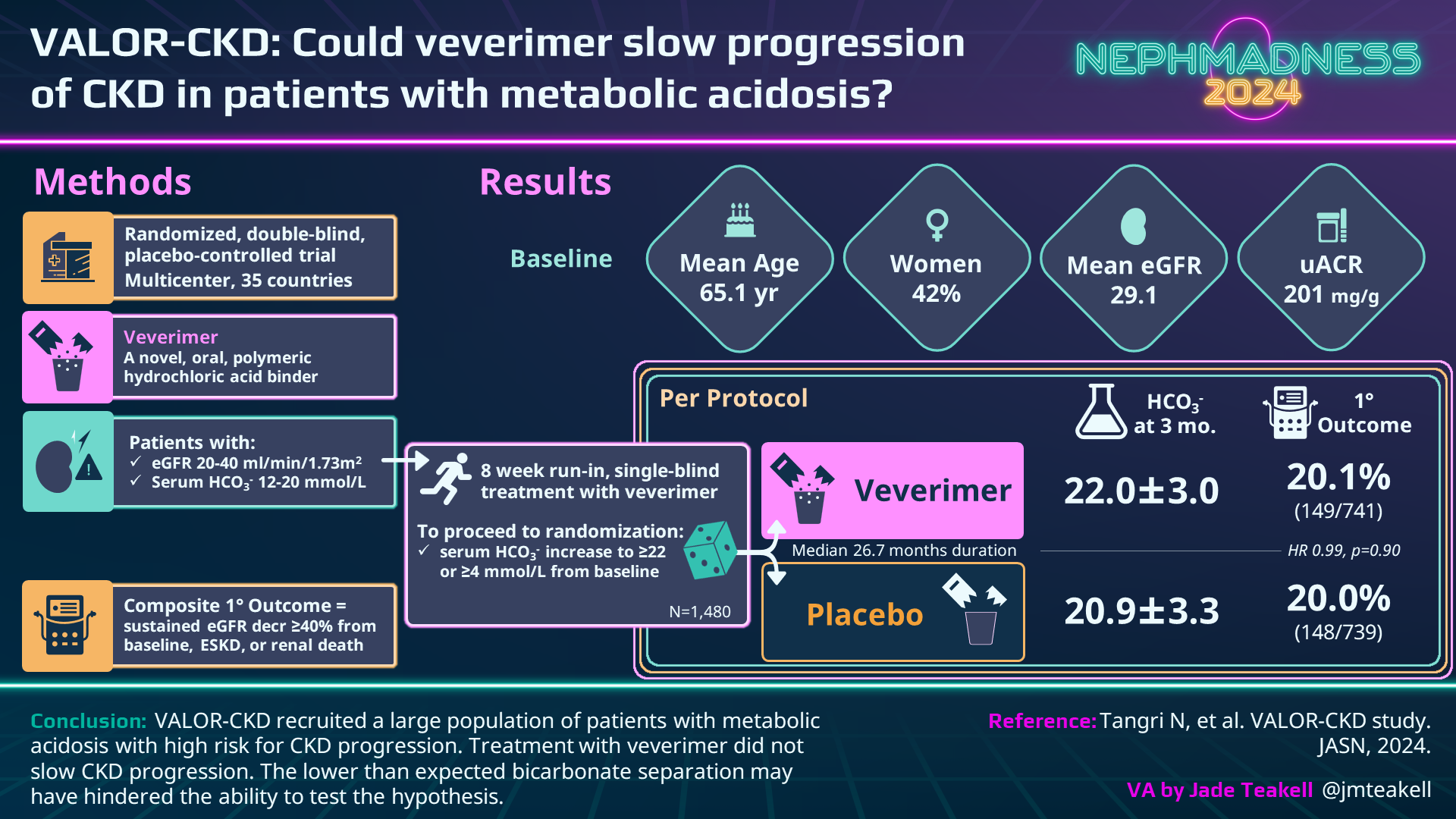
Lastly, let’s not forget that in 2019 a new player with an entirely new mechanism of action entered the fray. Veverimer is a non-absorbed polymeric selective binder of hydrochloric acid capable of removing the acid directly from the gastrointestinal lumen. As a once/day medication, it minimizes pill burden while eliminating acid (rather than neutralizing it) without the addition of sodium or potassium in patients with CKD. In 2019, a phase 3 study of veverimer showed that it was able to raise serum bicarbonate by 4 mmol/L in 59% of patients taking the drug compared to 22% in placebo over the course of 12 weeks. The primary side effects continued to be GI-related (nausea, diarrhea, constipation) in 13% of the treatment group. This study established the efficacy and safety of this novel drug, which led us to the VALOR-CKD trial which recruited many more patients (1,480) and aimed to establish hard endpoints such as progression to ESKD or 40% decline in eGFR from baseline. All patients had a 4-8 week run-in on veverimer and then were randomized to either continue the drug or switch to placebo. Unexpectedly, the patients who continued on the drug dropped their serum bicarbonate by 1.41 mEq/L after randomization, and the placebo group only dropped by 2.55 mEq/L, resulting in a very small difference between groups. Most importantly, there was no difference in the primary endpoint with treatment over the course of 3 years and the trial was terminated in May 2022 for administrative reasons leaving us with more questions than answers for the future of this drug.
Despite the vagary of veverimer, we know that metabolic acidosis is a common manifestation of more advanced CKD and may have important health consequences. Randomized studies assessing treatment of metabolic acidosis on hard endpoints are still needed, and hopefully this team shines a light on an often overlooked aspect of CKD management. Though chronic, and thus gradual in effect, the proposed negative manifestations of metabolic acidosis are too serious to not have our full attention and are a strong contender in this region.
COMMENTARY BY NAVDEEP TANGRI:
Show Me the Money!
Check out this podcast episode of The Curbsiders with Matthew Watto, Paul Williams, and NephMadness Exec Member Tim Yau:
#430 CKD, Metabolic Acidosis, Baking Soda vs Fruits and Veggies. It’s NephMadness 2024!
Check out this podcast episode of Critical Care Time with Nick Mark, Cyrus Askin, and NephMadness Exec Members Jeff Kott and Tim Yau:
#19 Metabolic Acidosis w/ NephMadness
Team 2: Metabolic Acidosis in AKI/ICU

Copyright: wk1003mike/Shutterstock
We have all been there – it’s 2 am on your last day of call and you get paged to consider emergent dialysis for a patient with AKI and severe metabolic acidosis. Despite a bicarbonate drip, the pH remains less than 7.1. You quickly go through your differentials while reviewing the labs, assess the patient, and you and your attending decide that a trial of high-dose CRRT is warranted. This may seem straightforward, but there is often a lot more to consider when it comes to metabolic acidosis in the ICU.
Metabolic acidosis in the inpatient setting is a common complication, particularly in the ICU. The epidemiology and characteristics are tricky to determine due to various pH limits used to define metabolic acidosis. Some disorders have a simple solution – for example, insulin might be the only thing needed to close the anion gap in diabetic ketoacidosis. For a patient with large volume GI losses, giving some bicarbonate-rich fluid is the easy answer. However, how often do we hear “just give them some bicarb” and wonder if this is really the right path? Simple chemistry suggests that adding a buffer to neutralize the excess protons is good for the patient, but is there any evidence to this practice? What happens if we add a buffer in a respiratory acidosis? Much like the basketball player who needs to wear the same lucky socks during March Madness, there seems to be a degree of superstition in the management of metabolic acidosis.
Interpreting Acid-Base Disturbances
Thinking back to acid-base during medical school, most of us were taught an elegant algorithm to help delineate what the cause of the acid-base disturbance is. Although our individual approach may vary, it’s important to go back to basics and approach each acid-base disturbance systematically along the lines of:
- What is the pH? → acidosis/alkalosis
- What are the bicarbonate and pCO2? → respiratory/metabolic
- Is there appropriate compensation from the other organ? → lung/kidney
Once in the category of metabolic acidosis, the next step is differentiating between a High Anion Gap Metabolic Acidosis (HAGMA) or a Non-Anion Gap Metabolic Acidosis (NAGMA). We calculate the AG by subtracting the serum chloride and serum HCO3 from the serum sodium. A normal gap is 10-12, but this is dependent on serum albumin (for every drop in 1 of albumin, the AG decreases by 2.5). The main difference between the two is that in a HAGMA there is an additional unmeasured anion that is responsible for the extra H+, whereas in a NAGMA there is generally a loss of bicarbonate (like in diarrhea or RTA). In the ICU and inpatient setting, it is predominantly HAGMA that causes concern as they can be life-threatening, whereas for the most part, NAGMA can be managed with conservative management.
Types of Metabolic Acidosis in the ICU
When it comes to HAGMA, some of us were taught MUDPILES, but GOLDMARK is a newer and more applicable mnemonic to remember the most common differentials. Since toxic alcohol ingestions (methanol, ethylene glycol) are on this list, it’s often a good idea to calculate an osmolal gap (measured serum osm – calculated serum osm) to determine if an osmotically active compound is driving the acidemia. The most common cause of metabolic acidosis in the ICU is lactic acidosis in the setting of hypoperfusion. Prospective trials have shown that severe acidemia with pH < 7.2 is present in up to 8% of ICU patients, and was associated with a 57% mortality.
NAGMA is a bit more straightforward, as ultimately it can be narrowed down to loss of HCO3 via the GI tract or the kidneys. An uncommonly discussed but commonly encountered cause is hyperchloremic metabolic acidosis in the setting of large volume administration of 0.9% NaCl. Physiologically, this is sometimes called a “dilutional acidosis” as the total volume expands with the same amount of bicarbonate molecules. (Ab)normal saline also has a pH of 5.5, although the development of acidosis with saline resuscitation is more complicated than this fact alone. This phenomenon was also demonstrated in surgical literature, where NS was compared to LR for post-op fluid resuscitation. Upon arrival to the SICU, the NS group had slightly lower pH (7.35 vs 7.4) and lower serum bicarbonates (21.1 vs 22.5) than the LR group.
Alkali Supplementation
Given how frequently we encounter metabolic acidosis, it makes sense that sodium bicarbonate is frequently given in the hospital, and in particular, in the ICU. But when is it correct to give sodium bicarbonate, and does it change outcomes? In the world of Rugby Union, there is a term called the dark arts of the scrum. A scrum is a battle of 8 vs 8 to get possession of the ball. To the viewer, everyone is huddled together in a scrum, but what actually happens to win the ball occurs under the surface and is only visible to the players. Depending on where you trained or who your coach was, players use different skill sets and techniques to win the scrum. The use of bicarbonate in metabolic acidosis in academia is similar, with different centers and providers following their own clinical gestalt (or even protocols) to determine when and how much bicarbonate to give.
Oftentimes, a pH of 7.20 is the cutoff for clinicians to decide to use bicarbonate. We can review some history for where this level comes from. In 1968, an animal study showed reduced left ventricular contractility at pH < 7.1 with infusion of lactic acid. Later animal studies in the 1990s showed that lower pH levels increased nitric oxide which provoked vasodilation and lowered blood pressure. However, at the same time, ICU trials looked at giving bicarbonate to patients with lactic acidosis and showed no change in hemodynamic stability despite improvements in pH. Some physiologic studies have examined the effect of bicarbonate for lactic acidosis and have gone so far as suggesting that alkali may increase intracellular acidosis although this is debatable. As far as clinical data goes, a recent meta-analysis looking at sodium bicarbonate repletion in the ICU showed no consistent evidence that sodium bicarbonate administration improved cardiac function.
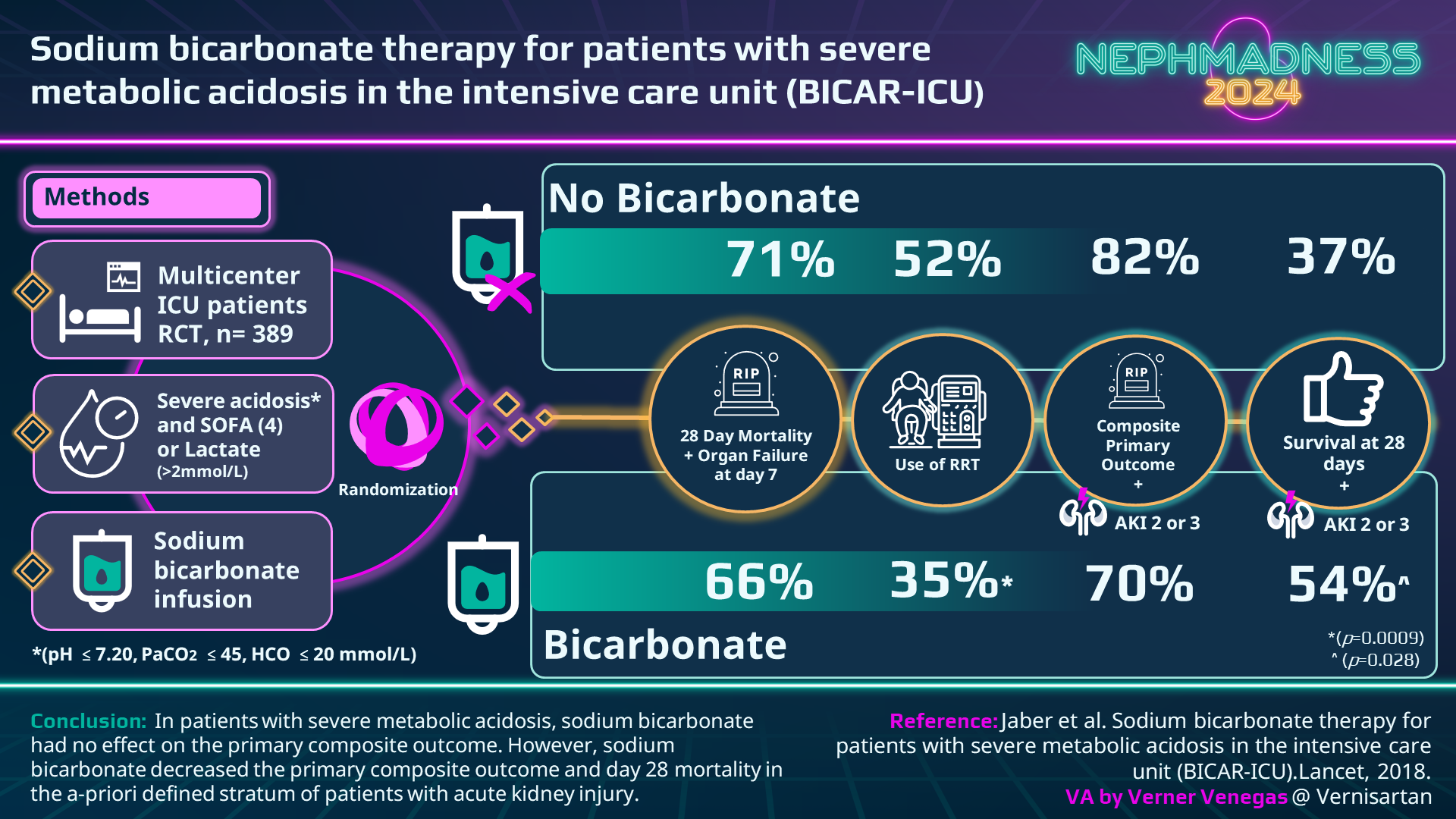
The most important (and one of the only) randomized controlled trial investigating the effects of sodium bicarbonate for severe metabolic acidosis was BICAR-ICU. This trial was conducted in 26 French ICUs, enrolled 389 patients with severe lactic acidosis (pH < 7.2, HCO3 < 20 mmol/L, lactate > 2 mmol/L), and aimed to look at whether administering sodium bicarbonate would improve the pre-specified primary outcome of 28 day survival compared to the control group of usual care. Despite the treatment group maintaining a higher pH, there was no difference in the primary outcome between the two groups. However, patients with AKI (AKIN score 2-3) experienced a mortality benefit and a reduced need for RRT, although the study was not powered for subgroup analysis.
It should be clear that efficacy data on the use of sodium bicarbonate for metabolic acidosis in the ICU is lacking, but is there any downside to giving it? Much like a fastball down the middle, it can be the saving grace that strikes out the batter (a.k.a resolves the acidosis) or it can be the pitch the batter was waiting for, crushing it to the left upper deck for a massive home run (a.k.a. complications of bicarbonate administration). Sodium bicarbonate is given orally or intravenously in a 1:1 molar ratio of bicarbonate which buffers the acid, but also of sodium. Especially when given IV, it is an isotonic crystalloid and causes volume expansion. In patients with sepsis in the ICU, this is usually a good thing, but we’ve all seen cases where this can go too far. Add in a sprinkle of heart failure, and you may second guess whether the liters you give may cause more harm than good. When given in more hypertonic solutions (e.g. the BICAR ICU trial gave 4.2% sodium bicarbonate solutions), it can lead to hypernatremia. Bicarbonate may also precipitate drops in potassium due to bicarbonaturia which drives potassium excretion, in addition to the more rapid intracellular shifting. Increase in systemic pH from bicarbonate administration will increase calcium binding to albumin and reduce the amount of free ionized calcium which may have cardiovascular consequences.
In addition to volume overload and electrolyte shifts, administration of sodium bicarbonate increases pCO2 via carbonic anhydrase and should not be given to correct respiratory acidosis. Alternative agents such as tris-hydroxymethyl aminomethane (THAM) have the buffering capability without producing CO2. Unfortunately, they are excreted by the kidneys, so patients with AKI can accumulate toxic levels of the drug which manifests as hyperkalemia, significantly limiting their clinical utility.
RRT for Treatment of Metabolic Acidosis
When bicarbonate has reached its limits in the treatment of metabolic acidosis, the tried-and-true method is of course, dialysis. At the most fundamental level, dialysis is simply fixing the acidosis by giving more bicarbonate. With hemodialysis or any other diffusive clearance such as SLED/PIRRT, the bicarbonate concentration in dialysate can be modified as needed (typically 35-38 mEq/L). With convective clearance (such as CVVH), the replacement fluid has a much higher bicarbonate concentration (typically 35 mEq/L) than the effluent. In many ways, dialysis is just a REALLY BIG bicarbonate drip, and now we also have the capability to not run into problems with volume overload or the other metabolic derangements as mentioned above. It should be mentioned that the classical dosing of 20-25 cc/kg/hour is a good place to start based on trials like ATN, but in profound acidemia with ongoing lactic acid production, higher doses are often required to deliver sufficient bicarbonate.
There are no unique trials looking at RRT initiation for metabolic acidosis – most of the data comes from other AKI-related RCTs in which metabolic acidosis with severe acidosis is one of several indications for RRT. Trials such as AKIKI and IDEAL-ICU (both French ICU trials) primarily looked at delayed vs early start of dialysis in patients with AKI and sepsis in the ICU. 21% of the AKIKI cohort and 13.4% of IDEAL-ICU patients started RRT with metabolic acidosis as the indication. Similarly, the STAART-AKI was a large international cohort with over 3,000 patients, and 16.6% of their patients met the criteria to start dialysis for severe metabolic acidosis.
So, whether you believe in the power of sodium bicarbonate or not, the debate on these topics will continue. Given the frequency, the confusion, and the literal life and death severity of the matter, metabolic acidosis in the inpatient setting deserves more respect. While the last few years of nephrology have focused on the excitement of improving CKD outcomes with SGLT2-Is and nsMRAs, some of us just want to interpret an ABG to make our day. Metabolic acidosis management in the ICU is like the blue chip player you recruit in the summer because of their experience. Hopefully, in the future we can draft an exciting new prospect to help further guide our management and improve outcomes for our patients.
– Executive Team Members for this region: Timothy Yau @Maximal_Change and Elena Cervantes @Elena_Cervants | Meet the Gamemakers
How to Claim CME and MOC
US-based physicians can earn 1.0 CME credit and 1.0 MOC per region through NKF PERC (detailed instructions here). The CME and MOC activity will expire on May 31, 2024.
Submit your picks! | #NephMadness | @NephMadness



Leave a Reply