#NephMadness 2020: Vaccines Region
Submit your picks! | NephMadness 2020 | #NephMadness | #VaccineRegion
Selection Committee Member: Ibironke Apata
Ibironke Apata is an Assistant Professor in the Renal Division at Emory University School of Medicine, Atlanta, GA. She is a Nephrology consultant for the Dialysis Team at the Centers for Diseases Control and Prevention. Her public health work focuses on infection prevention and antibiotic stewardship in the dialysis setting.
Special thanks to Mark S. Freedman and Raymond A. Strikas from the CDC, both of whom provided valuable input for this region.
Writer: Pascale Khairallah @Khairallah_P
Pascale Khairallah completed a general nephrology fellowship and is currently pursuing a kidney transplant fellowship at Columbia University Medical Center. She is interested in the relationship between mineral bone disorders and vascular health in patients pre- and post-kidney transplantation.
Competitors for the Vaccines Region
Influenza Vaccination vs Pneumococcal Vaccination
Live Vaccines in Transplant vs No Live Vaccines in Transplant

Copyright: Numstocker / Shutterstock
The Hepatitis B Success Story
Hepatitis B vaccination is a sure frontrunner in any competition mentioning vaccination and kidney disease. The NephMadness Executive Team thought it would be best to highlight the impressive results of Hep B vaccination while keeping our focus on other emerging members of the vaccination strategy to save lives of kidney patients.
What was the story of hepatitis B vaccination and nephrology?
First, you have to look at the virus itself. Hepatitis B virus (HBV) is highly infectious and can remain alive on surfaces for as long as 7 days. This makes it a significant threat to patients on hemodialysis (HD) who are exposed to dialysis equipment regularly. HBV exposure in patients on hemodialysis is particularly concerning because the infection becomes chronic in up to 60% of infected patients on dialysis, as compared to chronicity rates of less than 10% in healthy individuals.
In the 1970s, the prevalence of HBV in patients on hemodialysis and dialysis staff reached 7.6% and 0.9%, respectively. As a result, the Centers for Disease Control (CDC) introduced several infection control strategies to minimize the risk of HBV transmission in dialysis facilities:
- by 1983, isolating patients with a positive hepatitis B surface antigen (HbsAg) from patients with a negative HBsAg result
- testing for HBV routinely in patients on dialysis
- disinfecting dialysis equipment
These efforts resulted in a decrease in HBV prevalence to as low as 2.4% in patients on dialysis and 0.6% in the dialysis facility staff.
In the 1980s, the HBV vaccine was introduced, becoming the best line of defense against HBV infection in this population. Since the implementation of these HBV control practices, there has been only one published case (2016) of HD-related HBV transmission.

Algorithm for HBV vaccination and monitoring in dialysis patients. Figure 1 from Reddy et al, ACKD © National Kidney Foundation.
The use of intradermal (ID) administration of the HBV vaccine may improve seroconversion rates. In one study, where weekly ID HBV vaccine was administered for 8 weeks and was compared to intramuscular vaccination (IM) at weeks 1 and 8, seroconversion rates two months following completion of the vaccination courses were 79% for ID administration, as compared to 40% for IM administration. By 10 months, 55% of those who had received the IM vaccine (as compared to only 25% of those who had received the ID vaccine) had lost protective antibodies, defined as anti-HBs titer>10 IU/L. However, it was not clear if the mechanism of greater efficacy of ID vaccination was the cumulative effect of more injections, or the different route of administration.

Visual Abstract by @EricAu on Barraclough et al
Because seroconversion in response to HBV vaccination is much lower in end stage kidney disease (ESKD), it is best to vaccinate earlier. As CKD advances, the rates of seroconversion in response to the HBV vaccine series drop to 50% and patients on dialysis require higher doses. Consequently, both the US and UK guidelines recommend the higher dose/higher frequency vaccine schedule for patients on dialysis (either PD or HD).

Table 2 from Krueger et al, AJKD © National Kidney Foundation.
HBV vaccination is a major vaccine success story that improved outcomes of patients on dialysis. The CDC Advisory Committee on Immunization Practices (ACIP) recommends that all patients on dialysis receive an influenza and a pneumococcal vaccine. However, our success with these two vaccines has been suboptimal at best. Let’s take a look!
Influenza Vaccination vs Pneumococcal Vaccination
Influenza Vaccination
The benefits of vaccinating patients on dialysis against influenza have been well-demonstrated, as evidenced by up to 25% decreased rates of mortality in HD patients and up to 34% decreased rates of mortality in PD patients. Nephrologists and dialysis facilities, therefore, have made it part of their routine practice to administer the influenza vaccine to patients on dialysis. As a result, influenza vaccine rates have been steadily increasing, with 71% (Medicare participants) of patients on dialysis and having Medicare claims for influenza vaccination during the 2015-2016 season.
The weakened immune systems additionally result in an impaired immune response to vaccinations, such that the seroprotection rate in the dialysis population with standard influenza vaccines is 60% of that in the general population (47% vs 81%). Patients on dialysis who are younger than 60 years of age show better seroconversion rates as compared to patients on dialysis who are older than 60 years of age, though their seroconversion rates remain lower than those of the non-dialysis population.

Copyright: mihalec / Shutterstock
High-Dose Influenza Vaccine: Is it worth the cost?
Patients on dialysis have maladaptive innate and adaptive immune systems, making them susceptible to influenza and its complications. Strategies to enhance the immune response of patients on dialysis to improve their seroconversion rates are needed. The high-dose influenza vaccine first became available in the 2010-2011 flu season (the high-dose influenza vaccine contains 4 times the influenza antigen dose as the standard influenza vaccine). In 2014, a randomized clinical trial of adults aged 65 years and older in the general population showed that those receiving high-dose influenza vaccines had a reduction in influenza illness as compared to those receiving the standard dose influenza vaccine. As a result, some nephrologists started administering the high-dose influenza vaccine to all patients on dialysis in an effort to boost seroprotection rates.
In fact, in an observational study, receipt of the high-dose vaccine as compared to a standard dose quadrivalent vaccine was associated with fewer (barely) hospitalizations (HR 0.93; 95% CI 0.86-1.00; P=0.04) among patients on dialysis during the 2016-2017 influenza season. But the high-dose influenza vaccine was not associated with a significantly lower incidence of hospitalizations among patients on dialysis in the subsequent 2015-2016 season. Notable, neither vaccine reduced mortality rates in either year.
One study utilized the United States Renal Data System (USRDS) data to evaluate the effectiveness of the high-dose influenza vaccine as compared to the standard-dose vaccine within 225,215 patients (age 65 and up) on dialysis during 2010-2015 flu seasons. In this observational study, where only 2.6% of patients on HD received the high-dose vaccine, the risks for all-cause mortality and influenza- and pneumonia-coded hospitalizations were similar in patients receiving either vaccine.

Visual Abstract by @krithicism on Butler et al
How do these studies help us determine whether we should be choosing the standard vs high-dose influenza vaccine? Unfortunately, they do not answer the question. Standard dose influenza vaccine made up the large majority of the vaccines in the USRDS study. Additionally, immunity following influenza vaccination may last more than a single year. Finally, the variability in the severity of the influenza epidemic from year to year also makes it difficult to accurately assess the effectiveness of the vaccines, and likely affects the outcomes of studies comparing the vaccines.
The adjuvanted influenza vaccine is another option approved for individuals 65 years or older in the United States. The presence of the adjuvant MF59 (oil-in-water emulsion of squalene oil) improves the immune response to the vaccine. When given to Korean patients on dialysis (mean age 56 years +/- 12 years), the MF59-adjuvanted vaccines demonstrated better immunogenicity as compared to the standard dose influenza vaccine. The vaccine’s effectiveness in reducing hospitalizations has not been evaluated in the CKD or dialysis population.
Based on existing data, it is inconclusive whether there is a significant clinical benefit to using the high-dose vaccine in patients on dialysis. Given its safety, why not use the high-dose vaccine in everyone? High-dose influenza vaccines cost more than standard-dose vaccines, which could incur significantly higher costs if all patients on dialysis were to receive them. The high-dose vaccine additionally has more local and systemic side-effects (albeit all minor ones).
Pneumococcal vaccine
Pneumonia is a huge problem in patients on dialysis. In fact, USRDS data on 289,210 patients demonstrated that 21% of patients developed pneumonia within a year of starting dialysis (basically 1 in 5), 10% of which were receiving PD, between the calendar years 1996-2001. In fact, rates of pneumonia during the first year of starting dialysis were 59% higher in HD compared to PD. In the following 6-month period after pneumonia diagnosis, mortality rate was 73 per 100 patient-years, and the cardiovascular hospitalization rate was 65 per 100 patient-years.

Copyright: Jezper / Shutterstock
In contrast, the mortality rate was close to 15 per 100 patient-years, and the cardiovascular hospitalization rate close to 20 per 100 patient-years in patients who did not have pneumonia. These data support the need to protect patients on dialysis from pneumonia, with pneumococcal vaccination being a cornerstone to that effort.
The rates of pneumococcal vaccination in patients with CKD and those on dialysis are low. The prevalence of pneumococcal vaccination in the CKD population (non-ESKD) in the National Health and Nutrition Examination Survey (NHANES) from 1999 – 2004 ranges between 10 to 60%.

Vaccinated prevalence by vaccine indication between 1999 and 2004.No chronic kidney disease (CKD; ie, low risk) was estimated glomerular filtration rate (eGFR) ≥ 60 mL/min/1.73 m2 and urine albumin-creatinine ratio (UACR) < 30 mg/g. CKD with moderate risk was eGFR of 45 to 59 mL/min/1.73 m2 and UACR < 30 mg/g or eGFR ≥ 60 mL/min/1.73 m2 and UACR of 30 to 299 mg/g. CKD with high risk was eGFR of 15 to 44 mL/min/1.73 m2 regardless of UACR, eGFR of 45 to 59 mL/min/1.73 m2 and UACR of 30 to 299 mg/g, or UACR of 300 to 1,999 mg/g regardless of eGFR. Kidney failure was eGFR < 15 mL/min/1.73 m2 and nephrotic-range albuminuria was UACR ≥ 2,000 mg/g. Vertical lines indicate 95% confidence interval. Figure 2 from Ishigami et al, AJKD © National Kidney Foundation.
According to a study spanning 2006-2015, in the dialysis population, 32% of patients have Medicare claims for pneumococcal vaccination in the first year following dialysis initiation, 43% have claims after 2 years of dialysis initiation, and 68% have claims after 5 years. The rates of pneumococcal conjugate vaccine 13 (PCV13) vaccination have been increasing slowly since it was recommended in 2012. Less than 1% had received it in 2012, as compared to 7% in 2015.
As CKD is considered a condition resulting in an immunocompromised state, the CDC ACIP recommends that pneumococcal vaccine be given to patients with CKD as early in their disease as possible. As mentioned in the preamble on HBV and on team influenza vaccine, CKD impairs the innate and adaptive immunities, thereby decreasing seroconversion rates following vaccination. The same phenomenon is observed with the pneumococcal vaccines. Not only do patients have low seroconversion rates, but according to at least one study (though limited by the lack of opsonophagocytic assays), they have a quick decline in antibody titers as early as one year following vaccination.
There are currently two approved pneumococcal vaccines:
- Prevnar 13 or PCV13 which contains 13 different pneumococcal serotypes;
- Pneumovax 23 or PPSV23 which contains an additional 11 serotypes.
Between them, they have 12 common antigens. The administration of both vaccines offers better protection against pneumococcal pneumonia. The figure and table below summarize the recommended pneumococcal vaccine schedule in patients with CKD.
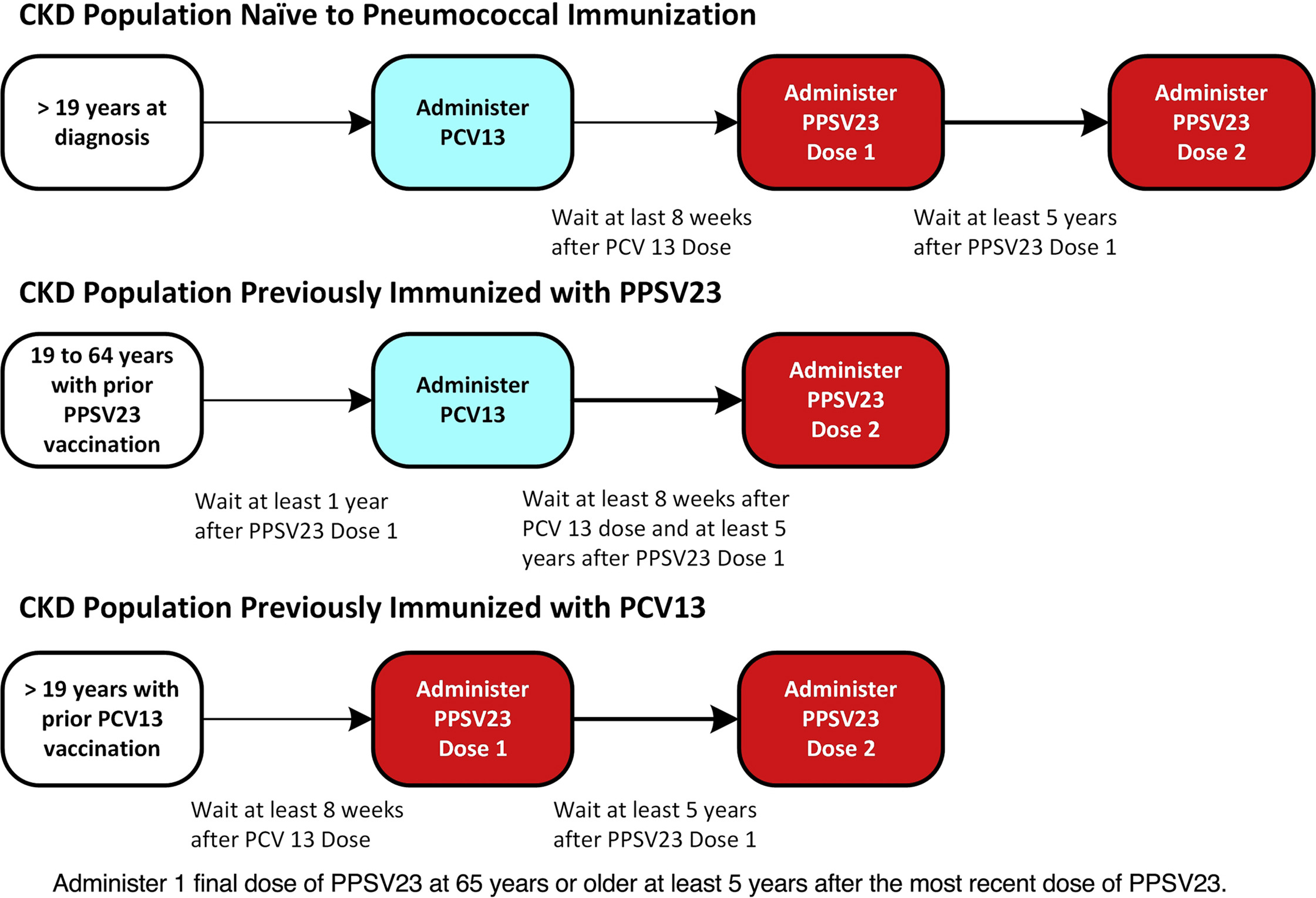
Pneumonia vaccine recommendations in patients with CKD. Figure 2 from Reddy et al, ACKD © National Kidney Foundation.

Table 3 from Krueger et al, AJKD © National Kidney Foundation.
There is room for much improvement when it comes to pneumococcal vaccination administration to patients with CKD and those on dialysis. New strategies to improve immunogenicity are also needed. Will the influenza vaccine or will the pneumococcal vaccine advance to the 2nd round? This may be one of the most difficult-to-predict matchups in the NephMadness tournament to date. Why, you ask? Just look at the figure below:

Survival curve summarizing deaths over the course of the 2005-2006 influenza season, by influenza and pneumococcal vaccination status. Figure 2 from Bond et al, AJKD © National Kidney Foundation.
Using data from 2005-2006, Bond et al evaluated the association between vaccination against seasonal influenza and pneumococcal disease and all-cause mortality in patients on dialysis. In 36,966 patients on PD and HD who had been on dialysis for greater than 1 year and with known vaccination status, both influenza and pneumococcal vaccines were independently associated with decreases in all-cause mortality [adjusted hazard ratio versus neither vaccination: 0.76 (95% CI, 0.71-0.82) for influenza and 0.81 (95% CI, 0.70-0.93) for pneumococcal disease alone]. The vaccines were additive, together decreasing all-cause mortality as compared to each alone [adjusted hazard ratio versus neither vaccination: 0.65 (95% CI, 0.61-0.70) for both vaccinations].
Live Vaccines in Transplant vs No Live Vaccines in Transplant
The American Society of Transplantation (AST) Infectious Diseases Community of Practice makes the following recommendations regarding vaccination before and after organ transplantation:

Non Live Vaccines adapted from Danzinger-Isakova et al AJT

Live Vaccines Adapted from Danzinger-Isakova et al AJT
Ideally, vaccination status should be closely evaluated in the pre-transplant period. Any deficient vaccinations should be completed by the time a patient is listed for transplantation. Despite clear recommendations from multiple societies emphasizing the important role of vaccines, transplant recipients remain under-immunized, both in the pre- and post-transplant periods.
In a single-center study of 362 patients on the kidney transplant wait-list between 2010 and 2014, 36% had completed Pneumovax vaccination, 55% had received an influenza vaccination, 7% received the zoster vaccine, and only 3% had a documented tetanus vaccination. Those currently receiving dialysis had a higher chance of receiving the HBV and pneumococcal vaccine as compared to those who were wait-listed but not on dialysis.
White race and receipt of other vaccines were factors associated with higher rates of Pneumovax vaccination. Among 1,352 kidney transplant recipients in three healthcare organizations in the United States between 1995 and 2005, 43% were vaccinated against influenza during the influenza season preceding the kidney transplant and 51% were vaccinated in the first full influenza season post-transplantation.
Finally, only 69% of 543 organ transplant recipients (477 single organ transplant and 139 stem cell transplant recipients) from the United States, Canada, and Spain admitted with influenza A or B between 2010 and 2015 were immunized against influenza during the year they were admitted.
As discussed in the first matchup, patients with kidney disease, particularly those receiving dialysis, have lower seroconversion rates in response to vaccinations. In addition, they lose immunity faster than patients without kidney disease. Therefore, even if nephrologists ensure that their patients have received appropriate vaccinations and even if antibody titers are adequate during the pre-transplant evaluation period, patients may lose immunity by the time they are transplanted. Consequently, patients may require immunizations post-transplantation.
Can patients get live vaccines post-transplantation or are they restricted to inactivated vaccines, as suggested by the CDC ACIP recommendations?
Live Vaccines in Transplant

Copyright: Roman Silantev / Shutterstock
First off, it is important to recognize that the use of live vaccines in patients with a functional transplant is controversial. They have been listed as contraindicated because of the risk of causing disseminated disease because of the immunosuppressed state. Not vaccinating transplant recipients with live vaccines was considered an acceptable practice for many years, as the prevailing thought is that they would be protected via herd immunity secondary to the high rates of vaccination in the general population.
But this changed in recent years, in 2007, the anti-vaccine movement started gaining traction, and by 2013, measles outbreaks were appearing. Since then, measles cases have been increasing globally, with more measles cases reported worldwide in 2019 than in any year since 2006. Mumps outbreaks and varicella outbreaks are occurring with similar frequency. Non-immunized patients who are transplant recipients are no longer as well protected from contagious, vaccine-preventable diseases.
There are no high-quality studies establishing the safety of live vaccines in adult organ transplant recipients. A systematic review of 64 published trials, observational studies, and case reports aimed to evaluate the safety of live vaccination in immunocompromised patients, including those with solid organ transplants. The systematic review encompassed 21,082 patients from 18 different countries. Of those, 339 patients had solid organ transplants, 62 of whom were kidney transplant recipients who had received MMR, varicella, yellow fever, and Bacillus Calmette–Guérin vaccines post-transplantation. Local and systemic vaccine-associated adverse reactions were observed in 61 of the 339 (18%) solid organ transplant recipients. Of those 61 with adverse reactions, 16 (4.7% of the total 339) had a vaccine-related infection, with 14 of the 16 developing varicella within 2-8 weeks following varicella vaccination. In all 14 patients who developed varicella, the varicella was moderate, without long-term complications. The other two patients who developed infection had transient swelling of the parotid glands after mumps immunization. Three liver organ rejections were reported after varicella vaccination. Of note, all adverse reactions were in liver, intestine, and heart transplant recipients. None were reported in 62 patients with a kidney transplant.

Visual Abstract by @jbda19 on Croce et al
Despite the lack of strong evidence supporting the safety of live vaccines in kidney transplant recipients, a pediatric transplant consensus meeting provided general guidelines that could be extrapolated to the adult transplant population.
- T-cell depleting agents such as anti-thymocyte globulin or alemtuzumab used for induction or treatment of some rejection episodes can have a lasting effect on T-cells for at least 1 to 2 years post-transplantation. Live vaccines are therefore not recommended until at least a year has elapsed since the last treatment with these agents. These recommendations extend to novel biologic agents which lead to T-cell dysfunction of unknown duration.
- Rejection episodes are also treated with high doses of immunosuppressive medications. Therefore, it is recommended that live vaccines be withheld for at least 2 months following rejection therapy.
- Cytomegalovirus and Epstein-Barr virus infections generally reflect a state of T-cell dysfunction. As such, live vaccines should be deferred until these infections have resolved.
- Studies establishing the safety of using live vaccines in patients who have received mycophenolate mofetil or rituximab are lacking.
Even if patients have had no recent rejection, no infection, and are several years post-induction, they should be clinically stable in order to receive a live vaccine. Importantly, their maintenance immunosuppression regimens should be stable as well. Per the pediatric consensus meeting, tacrolimus trough levels should be maintained below 8 ng/mL, and cyclosporine levels should be maintained below 100 ng/mL. If on prednisone, the dose should be less than 20 mg per day.
There are significant knowledge gaps in the area of vaccination pre- and post- kidney transplantation:
- When should we assess the immune status and vaccination titers pre-transplantation?
- What level of immunosuppression is safe for administration of inactivated vaccines?
- Which live vaccines are safe?
- What is the optimal time to administer a vaccine following rituximab and newer immunosuppressants?
- How often should we check titers post-transplantation?
- Are booster doses required post-transplantation?
No Live Vaccines in Transplant

Copyright: Roman Silantev / Shutterstock
Inactivated vaccines in all forms: killed whole-organism virus, recombinant virus, subunit or split-virus, toxoid, polysaccharide, and polysaccharide protein-conjugate vaccines can be administered to kidney transplant recipients. No doubt the mantra of Team No Live Vaccines in Transplant are that risks of disease dissemination caused directly by the vaccine is far worse than the immunity that it would potentially confer. Although we are seeing a resurgence in measles and mumps, they are only occurring in isolated clusters. The current risk/benefit ratio is firmly on the side of risk when it comes to Live Vaccines. Thus, Team No Live Vaccines is surely the team to beat in NephMadness.
Several factors and clinical situations affect the timing of vaccinations. Following induction immunosuppressive therapy, the immune response to vaccinations will be blunted. As a result, it is generally recommended to delay vaccination for at least 6 months post-induction of immunosuppression. Similarly, patients who have quantitative B-cell deficiencies and who are receiving rituximab, other anti-B cell antibodies, or immunoglobulin therapy should not receive inactivated vaccines until at least 6 months after completion of their treatment. This recommendation reflects concerns about the low effectiveness of vaccines in states of quantitative or qualitative T- and B-cell deficiencies. The efficacy of inactivated vaccines administered more than 6 months after induction therapy or B-cell targeted therapy, or at any time after newer immunosuppressive agents, is unpredictable. As a result, measuring antibody titers may be warranted to determine the need for a booster dose.
Additionally, the efficacy of vaccination in kidney transplant recipients may be influenced by the type of maintenance immunotherapy they are receiving. Older studies suggest that patients on azathioprine may have higher seroconversion rates as compared to those on cyclosporine. In a more recent study, organ transplant recipients on mTOR inhibitors had lower vaccine response as compared to recipients receiving cyclosporine, mycophenolate mofetil, or corticosteroids.
The influenza vaccine is an exception to the above rules. Influenza confers such a high morbidity and mortality to transplant recipients that it is recommended that the vaccine be given as early as 1 month post-transplantation in pandemic situations or as early as 3 months post-transplantation if the transplant occurred during the influenza season. Some studies have raised concerns that the administration of the influenza vaccine so early post-transplantation can cause immune upregulation and the development of de novo donor specific antibodies. Nevertheless, follow up studies indicated that the vaccine does not increase the risk of rejection in the organ transplant population. In fact, the administration of the influenza vaccination during the first year post-transplantation resulted in lower allograft loss and death. The Pneumovax 23 vaccine was not shown to induce an alloresponse in kidney transplant recipients and, as such, is not associated with rejection.
More recently, there has been increasing interest in the prevention of herpes zoster because of the significantly higher incidence of zoster infection in immunocompromised individuals as compared to immunocompetent individuals. In 2017, the FDA approved Shingrix for the prevention of zoster in adults ≥50 years of age. Shingrix is a non-live recombinant subunit vaccine, making it the first zoster vaccine that could be given to individuals who are immunocompromised. The vaccine showed promise in 121 bone marrow transplant patients, resulting in good seroconversion rates with positive titers persisting for at least one year post-vaccination. One patient developed pneumonia 105 days following the vaccine; this event was considered vaccine-related and was the only documented adverse event.
Recently, the results were published of a phase 3, randomized, observer-blind, placebo-controlled, multicenter trial, conducted in 9 countries between March 2014 and April 2017, evaluating the immunogenicity and safety of Shingrix in kidney transplant recipients. Among 264 patients enrolled in the study (132 received Shingrix and 132 received placebo), 80% of recipients developed adequate vaccine response rates. Of those who received the vaccine, 87% reported local site pain, 49.6% reported myalgias, and 16% reported fevers (as compared to 8.3%, 23.5% and 3.8% respectively in the placebo group). Throughout the entire study, 4 (3.0%) and 7 (5.3%) biopsy-proven rejections occurred in the Shingrix and placebo groups, respectively. Four (3.0%) recipients in each group had an increase in creatinine by the end of the study. The results of this study are promising, but larger and longer-term studies are needed to prove the safety of Shingrix in kidney transplant recipients.

Visual Abstract by @krishnadoctor1 on Vink et al
– Executive Team Member for this region: Matthew Sparks, AJKD Social Media Advisory Board member. Follow him @Nephro_Sparks.
How to Claim CME and MOC
US-based physicians can earn 1.0 CME credit and 1.0 MOC point for reading this region.
- Register/log in to the NKF’s Professional Education Resource Center (PERC). If you select “Physician” in the drop-down menu during registration, the ABIM ID will pop up – make sure to complete this during registration to receive MOC points after course completion.
- Review the activity, disclosure, and accreditation information.
- Click “Continue” and review Course Instructions.
- Complete Post-Test. Please note: By selecting “Yes” to the participation questions for each region, the corresponding Post-Test questions will appear. Click “Save Draft” to save your responses and finish later. When you are ready to submit your answers, click “Preview” to review all responses, then click “Submit.”
- Click “Next” to complete the Evaluation form, then click“Submit.”
- Claim 1.0 CME credit and 1.0 MOC point per region (up to 8.0 total for 8 regions of NephMadness).
- Save/print your certificate.
The CME and MOC activity will expire on June 13th, 2020.


Leave a Reply