Y SeX and Gender Differences Matter in CKD: Understanding the Impact on Kidney Function Decline
Dr. Jenny Pan is an Associate Professor at Baylor College of Medicine and the Michael E. DeBakey VAMC in Houston, TX. At MEDVAMC, she serves as the Director of the kidney living donor program. Her research interests include the role of mitochondrial anti-oxidant pathways in acute kidney injury and elucidating mechanisms for observed sexual dimorphism in kidney disease.
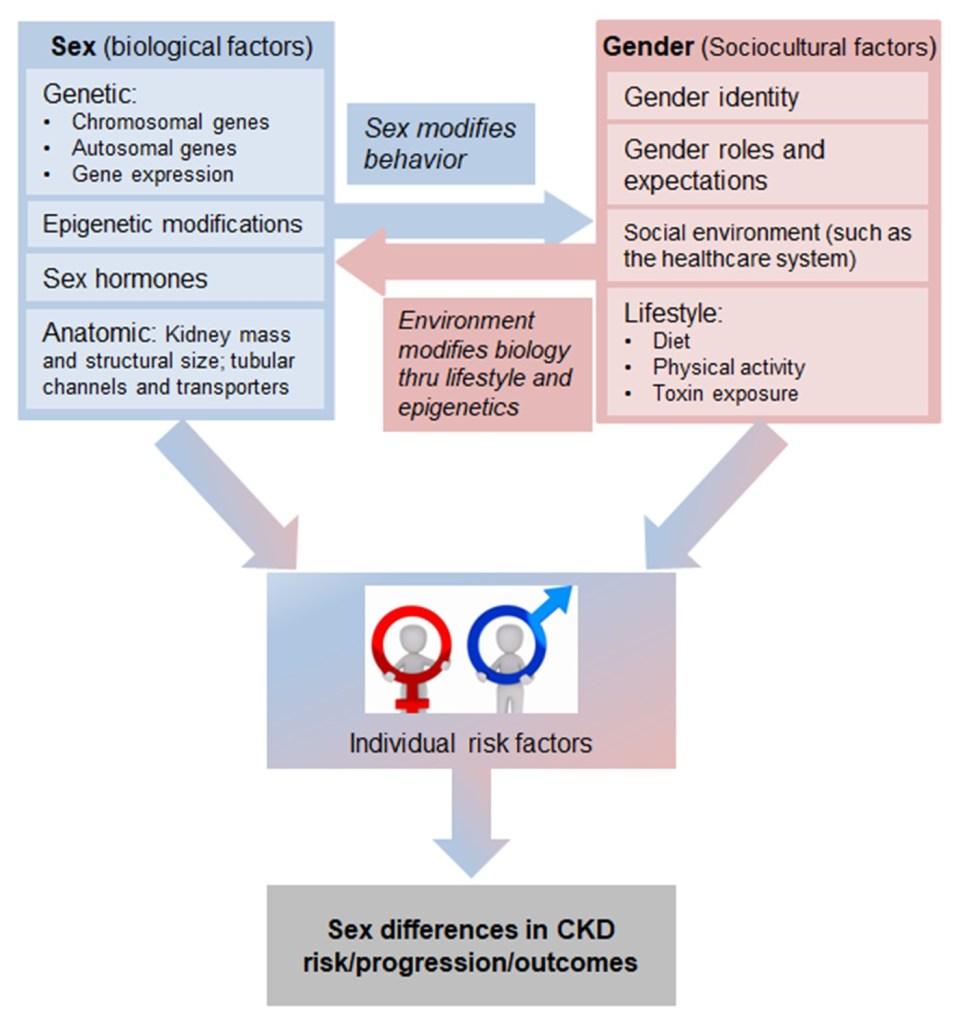
“Gender medicine,” a term first introduced in the late 1990s, is the study of how health is influenced by sex- and gender-based differences. Sex-based difference are biological and include differences in chromosomes, gene expression, and hormones. Gender-based differences are sociocultural and include differences in factors such as lifestyle, environmental influences and exposures, and sociocultural roles and expectations. Males/men and females/women have differing experiences of disease in a variety of domains including prevention, clinical manifestation, diagnostic and therapeutic approaches, prognosis, psychosocial effects, and interactions with the healthcare system. Sex and gender have a complex interplay, interacting, influencing, and even opposing each other to contribute to disparities between males and females in CKD risk, progression, and outcomes (Figure).
Over the past few decades, many studies have reported differences between males and females in terms of CKD burden, progression, and outcomes. These include findings that females have an increased incidence of CKD but are less likely than their male counterparts to progress to ESKD. Other key differences were nicely summarized by Ha and Hockham in a prior AJKD blog commentary. Biological differences that may contribute to the observed sexual dimorphism in CKD include:
- Anatomic: studies have demonstrated that total kidney mass and the cortex and proximal tubules are larger in males than in females. In contrast, the medulla seems to be larger in females. Thus, the impact of a declining GFR on a female’s risk of developing CKD complications, progressing to kidney failure and mortality may not be the same as for a male.
- Renal Transporters: major sex differences in both the expression and abundance of electrolyte, acid–base, water and organic solute transporters have been documented. For example, there is greater expression of sodium-glucose cotransporter 2 (SGLT2) and Na+-K+-2Cl−cotransporter (NKCC2) in females. These differences may affect the efficacy and optimal dosing of medications commonly used in the management of CKD (e.g., SGLT2-inhibitors, diuretics) in males versus females. It has been reported that women experience adverse effects from medications at twice the rate of men.
- Sex Hormones: in animal studies, androgens have been shown to exert a deleterious effect within damaged kidneys, whereas estrogen exerts a protective effect in terms of fibrosis, inflammation, oxidative stress, and modulation of the RAS system. There is also data supporting the hypothesis that female hormones are protective again CKD progression in humans. Kattah et al found that females with surgical oophorectomy prior to the natural age of menopause are at an increased risk of developing CKD as compared to females with no oophorectomy.
Gender-based sociocultural differences that may contribute to the observed sexual dimorphism in CKD include:
- Women are more likely to choose conservative care for their kidney failure, and are less likely to be listed for and to receive a transplant (including pre-emptive transplant). This may contribute to differences in progression to kidney failure in studies that use initiation of KRT as a surrogate endpoint.
- Lifestyle: some studies suggest that men may have a higher risk of kidney function decline due to lifestyle factors such as higher rates of smoking and injuries, decreased compliance with CKD-specific dietary restrictions, and occupational exposures.
- Social Determinants of Health: studies demonstrate that globally, more women live in extreme poverty, live with food insecurities, have lower incomes and limited access to secondary education compared with men, which all contribute to barriers in access to appropriate healthcare.
However, the distinct contributions of biological sex and the sociocultural dimension of gender to the manifestations and outcomes of CKD remain inadequately understood, and current guidelines do not provide sex-specific recommendations. In the study by Sullivan et al recently published in AJKD, the authors explored the relationship between sex and various cardiometabolic risk factors and how they influence the decline in kidney function over time in a large population-based cohort. The study included 1.1 million adults living in Wales, UK, who had at least three eGFR measurements between 2013 and 2020 reported within the Secure Anonymised Information Linkage Databank. CKD risk factors studied included hypertension, diabetes, coronary artery disease (CAD), heart failure, peripheral vascular disease (PVD), atrial fibrillation, stroke or transient ischemic attack (TIA), smoking and socioeconomic status. The primary outcome was the yearly decline in eGFR, while the secondary outcome was the risk of incident kidney failure, defined as the need for long-term kidney replacement therapy (KRT) and/or sustained eGFR less than 15 mL/min/1.73 m². Their results demonstrated that the average decline in eGFR was similar in males and females age ≤73. After age 73, eGFR decline was faster in males than females. These findings would appear to contradict the hypothesis that female sex hormones exert protective effects in the kidney as menopause occurs well before age 73 in females. However, one confounding factor is that in females, CKD is associated with hormonal dysregulation (including lower systemic estradiol levels), menstrual irregularities and premature menopause. This study did not include data regarding reproductive history, hormone levels or the use of oral contraceptives and hormone replacement therapy. Consistent with previous studies, Sullivan et al found that the risk of kidney failure in males was almost double that in females (unadjusted HR 1.90, 95% CI 1.77-2.04). The association between male sex and kidney failure was driven more by KRT initiation than persistent eGFR <15 mL/min/1.73 m². The authors hypothesize this may be related to factors reported in other studies such as higher prevalence of cardiovascular disease in males with CKD necessitating KRT for symptom management (e.g., pulmonary edema) or females being more likely to choose conservative care.
Further analysis was performed to examine differences in the impact of common CKD risk factors on eGFR decline in males versus females. The authors reported that before and at the age of 73, the presence of risk factors was not associated with significant differences in kidney function decline between males and females. Risk factors with the greatest rate difference were PVD (males: -1.04 mL/min/1.73m2 per year vs. females: -0.87 mL/min/1.73m2 per year) and diabetes (males: -1.15 mL/min/1.73m2 per year vs. females: -1.06 mL/min/1.73m2 per year). After 73 years of age, the presence of each risk factors studied was associated with sex differences in kidney function decline, especially heart failure (males: -1.22 mL/min/1.73m2 per year vs. females: -0.87 mL/min/1.73m2 per year) and smoking (males: -1.58 mL/min/1.73m2 per year vs. females: -1.27 mL/min/1.73m2 per year). Interestingly, the risk factors that contributed most to eGFR decline varied between males and females. While smoking and diabetes were the top two risk factors in both sexes, the third most impactful risk factor in females was socioeconomic deprivation and peripheral vascular disease in males.
As the authors point out, there are limitations to this study including: 1) the population was predominantly White (96%), older and had more comorbidities (as people excluded due to insufficient lab values tended to be younger with fewer health conditions) limiting its generalizability; and 2) data for albuminuria (a key risk factor for kidney function decline) was not available. Despite these limitations, the study advances our understanding about sex/gender differences in CKD by demonstrating that there are sex differences in the association of established risk factors with CKD progression. Key findings suggest that clinical management strategies for CKD should consider sex/gender-specific differences when developing interventions to reduce risk factors. For example, males, particularly those with heart failure and smoking habits, may require more aggressive monitoring and intervention. And while consideration of socioeconomic factors in developing care plans is important for all patients, it is imperative in women, especially those living in socioeconomically deprived areas. This study joins a large body of literature highlighting the need for additional research to explore the influence of biological and sociocultural differences between men and women on CKD outcomes, and the development of medical innovations that account for and leverage these differences. Even with the mandated inclusion of women in NIH-funded studies in 1993, sex and gender continue to be inconsistently reported or not included in the study design or analysis of clinical trials in nephrology. Efforts to bring sex and gender into medical research, education and practice are necessary and a fundamental step towards precision medicine in kidney disease, which will improve care for both men and women.
-Post prepared by Jenny Pan
To view Sullivan et al (Open Access), please visit AJKD.org:
Title: Sex and the Relationship Between Cardiometabolic Risk Factors and Estimated GFR Decline: A Population-Based Cohort Study
Authors: Michael K Sullivan, Jennifer S Lees, Brenda M Rosales, Rachel Cutting, Melanie L Wyld, Mark Woodward, Angela C Webster, Patrick B Mark, Nicole De La Mata
DOI: 10.1053/j.ajkd.2024.05.007


Leave a Reply